ABSTRACT
Background
Fomepizole is a competitive alcohol dehydrogenase inhibitor used for the treatment of ethylene glycol and methanol poisoning. We evaluated the safety and effectiveness of fomepizole in patients with ethylene glycol or methanol poisoning in Japan.
Research design and methods
This retrospective post-marketing surveillance study conducted in Japan registered patients who received fomepizole intravenous infusion per the package insert (January 2015−June 2022). Endpoints included adverse drug reactions/infections (ADRs), arterial blood pH, and treatment outcomes.
Results
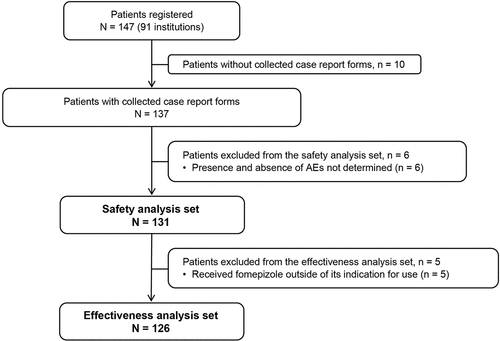
Of 147 patients registered (91 institutions), 131 and 126 were included in the safety and effectiveness analysis sets, respectively. Mean age was 43.6 years, and 66.4% were male. Mean time from poison ingestion to treatment was 15.1 hours; 66.4% received concomitant hemodialysis. No serious ADRs were reported. ADRs were reported in seven patients; the most-reported ADR was vomiting (2.3%). Seven patients died, 105 survived without sequelae, and 19 survived with sequelae. Most common sequelae were renal failure or renal dysfunction. Mean arterial blood pH increased to 7.4 by 4 hours of treatment, remaining stable for 24 hours post-treatment.
Conclusions
Fomepizole is well tolerated and helps improve clinical outcomes in patients with ethylene glycol or methanol poisoning in Japan.
Trial registration
Japanese Pharmaceutical Information Center (JapicCTI-152817).
1. Introduction
Ethylene glycol and methanol are readily available in many household products, such as antifreeze and cleaning solutions; poisoning from these alcohols can occur through unintentional misuse and accident, as well as intentional abuse and attempted suicide [Citation1]. Ethylene glycol and methanol are metabolized by alcohol dehydrogenase (ADH) and aldehyde dehydrogenase to their toxic metabolites, glycolic acid, glyoxylic acid, and oxalic acid (ethylene glycol) [Citation2], and formaldehyde and formic acid (methanol) [Citation3]. Consumption of these alcohols causes an increase in osmolar gap [Citation3,Citation4], and formation of their toxic metabolites causes metabolic acidosis and increased anion gaps [Citation3–5] and is responsible for various clinical symptoms. These symptoms include acute kidney injury, coma, seizures, and cardiovascular failure in ethylene glycol poisoning, and visual impairment, chest pain, and dyspnea in methanol poisoning [Citation6]. These toxic alcohol poisonings are associated with significant morbidity and mortality [Citation5,Citation7] and pose serious concerns worldwide [Citation8]. In the United States, hospitalization rates range approximately between 30% and 40% [Citation8,Citation9], and high mortality rates exceeding 30% are also reported globally when diagnosis and treatment are delayed [Citation10,Citation11]. Therefore, early detection of poisoning and initiation of treatment are essential to prevent sequelae and improve survival.
Fomepizole is a potent competitive ADH inhibitor that blocks the ADH-mediated metabolism of ethylene glycol and methanol and reduces the formation of their toxic metabolites [Citation12]. Two multicenter prospective clinical trials in the United States have shown the safety and efficacy of fomepizole treatment for ethylene glycol and methanol poisoning [Citation13,Citation14]. In patients with ethylene glycol poisoning, fomepizole progressively decreased plasma glycolate concentration down to around 0 mg/dl by 24 hours, and progressively increased arterial pH and serum bicarbonate concentration to their normal levels [Citation13]. In patients with methanol poisoning, initiation of fomepizole treatment reduced formic acid concentration and simultaneously resolved metabolic acidosis [Citation14]. In both studies, most patients survived from the poisonings; few adverse events (AEs) attributable to fomepizole were observed [Citation13,Citation14]. However, both studies had a small sample size of < 20 patients, and the results may not be generalizable to patients of all ethnicity types.
Currently, fomepizole is approved worldwide for the treatment of ethylene glycol and methanol poisoning and is recommended as an effective first-line antidote [Citation5], with a superior safety profile to ethanol [Citation15]. In 2010 in Japan, fomepizole was identified as an unapproved/off-label use drug of high medical importance, which led the Japanese Ministry of Health, Labour and Welfare to request the development of the drug domestically; fomepizole was subsequently approved in Japan in 2014. Nonetheless, information on the safety and effectiveness of fomepizole in the clinical setting is still limited [Citation16], particularly in Japan. To date, reports on fomepizole treatment for ethylene glycol and methanol poisoning in clinical practice in Japan consist of only case studies of a small number of patients [Citation17–20]. Given that these poisonings are an ongoing concern, and because there are no diagnostic or treatment guidelines currently available, understanding the safety and effectiveness of fomepizole in Japan will provide valuable information to the physicians treating these poisonings and will supplement the currently scant evidence of fomepizole in Japan.
This post-marketing surveillance (PMS) study aimed to evaluate the safety and effectiveness of fomepizole in patients with ethylene glycol or methanol poisoning in real-world clinical practice in Japan.
2. Patients and methods
2.1. Study design
This was a retrospective PMS study conducted in Japan between 25 January 2015 and 30 June 2022 in patients who received fomepizole (4-Methyl-1H-pyrazole) intravenous (IV) infusion for ethylene glycol or methanol poisoning. Patients were enrolled between 27 January 2015 (i.e. start of fomepizole IV marketing in Japan) and 31 January 2022 using a central enrollment procedure. Patient enrollment data were collected by the investigators at each study site using the contact form enclosed in the drug product packaging, which was sent to the Central Enrollment Center. Patient data were collected using a case report form completed and submitted by the investigator to the study sponsor within approximately 1 month of the end of the observation period, defined as the period between baseline (i.e. the start of fomepizole treatment) and hospital discharge or until transfer to another department. The study was conducted in compliance with the GPSP Ordinance (Good Post-marketing Study Practice; Ministerial Ordinance No. 171 of the Ministry of Health, Labour and Welfare dated 20 December 2004) and other relevant regulatory requirements. In accordance with these regulations, neither independent review board and ethics committee approvals nor patient consent were required. All data used in this study were anonymized. The study was registered at the Japanese Pharmaceutical Information Center (identifier: JapicCTI-152817) and at ClinicalTrials.gov (identifier: NCT02415712).
2.2. Study population
All institutions that received and prescribed fomepizole and agreed to participate in the PMS study were included. Patients who received fomepizole IV between 27 January 2015 and 31 January 2022 were eligible for this study.
2.3. Treatment protocol
Fomepizole was administered according to the dosage and administration described in the package insert [Citation21] and was administered every 12 hours by IV infusion over a period of at least 30 minutes at an initial dose of 15 mg/kg, followed by 10 mg/kg for the second dose and up to the fifth dose, then 15 mg/kg for the sixth and subsequent doses. When combined with hemodialysis, fomepizole was administered based on the timing of the hemodialysis. At the start of hemodialysis, fomepizole was administered immediately before starting dialysis if at least 6 hours had elapsed since the previous fomepizole infusion; if less than 6 hours, fomepizole was not administered. During hemodialysis, fomepizole was administrated every 4 hours. At the end of hemodialysis, fomepizole was administered at 50% of the standard dose if 1–3 hours had elapsed since the previous fomepizole infusion; if more than 3 hours, the standard fomepizole dose was administered immediately after the completion of hemodialysis and if less than 1 hour, fomepizole was not administered. After the end of hemodialysis, fomepizole was administered every 12 hours from the previous fomepizole infusion.
2.4. Survey items
At the time of enrollment, information on sex, age at baseline, reason for fomepizole use, and start date of fomepizole treatment was collected. Data collected on patient demographics and baseline characteristics included diagnosis (type of poisoning), hypersensitivity factors (presence and absence), concurrent diseases (presence and absence, and details), previous medical history (presence and absence, and details), height, body weight, alcohol consumption at the time of poisoning (presence and absence), pregnancy (for females only), consciousness level, and circumstances of poisoning (e.g. name of ingested substance and amount, time and route of ingestion, reason ingested). All information was collected at baseline except for diagnosis information, which was recorded during the observation period.
Information on treatment details was collected during the treatment period, defined as the period from baseline to the end of fomepizole treatment, and included administration status (number of infusions, dosage [per infusion and per body weight], treatment duration, reason for treatment discontinuation). Information on concomitant pharmacological treatments and non-pharmacological interventions and procedures (presence and absence, details, including drug/therapy name[s], and reasons for use) was collected from the time of poisoning to the completion of the final fomepizole infusion. Vital signs, laboratory test results, and poisoning and toxicity test results were collected at baseline and at 0.5, 4, 8, 12, 16, 20, and 24 hours after baseline, then every 12 hours, at completion of the final infusion, and at 12 and 24 hours after completion of the final infusion. Poisoning and toxicity test results collected were arterial blood pH, arterial partial pressure of carbon dioxide [PaCO2], arterial partial pressure of oxygen [PaO2], bicarbonate ion, base excess (BE), anion gap, concentrations of blood ethylene glycol, blood glycolic acid, blood methanol, blood formic acid, and osmolar gap. Information on toxic symptoms (presence and absence) was collected at baseline and at the end of fomepizole treatment, and information on outcomes (safety and effectiveness: survived without sequelae, survived with sequelae, death, including sequelae details) was collected at hospital discharge or at the time of transfer to another department.
Details of AEs – including presence and absence, AE terms, date of onset, seriousness, and reason for the assessment as serious, reason for fomepizole discontinuation, causal relationship with fomepizole (and its rationale if the event was assessed to be ‘not related’) – were collected from baseline to 24 hours after completion of the final fomepizole infusion. AEs were defined as any unfavorable and unintended signs (including abnormal laboratory findings, symptoms, or disease) occurring after the administration of fomepizole, which may or may not be considered related to fomepizole; abnormal worsening of the poisoning (e.g. worsening beyond the expected natural course of the poisoning) was assessed as an AE. If the AE outcome was assessed to be ‘not recovered/not resolved’ or ‘unknown,’ or the causal relationship was deemed ‘not evaluable,’ the event was to be followed by the investigators at each study site for as long as possible as requested by the sponsor.
2.5. Endpoints
Safety endpoints were the incidences of AEs and adverse drug reactions/infections (referred to as ADRs herein), serious AEs (SAEs) and serious ADRs, and ADRs identified in the safety specifications. ADRs were defined as all AEs except those with a causal relationship of ‘not related’ to fomepizole as assessed by the investigator. The important identified risk was anaphylaxis (Medical Dictionary for Regulatory Activities [MedDRA] preferred term [PT]: anaphylactic reaction, anaphylactic shock, anaphylactoid reaction, and anaphylactoid shock), and important potential risks were central nervous system disorder (MedDRA system organ class [SOC]: nervous system disorders) and fetal disorder due to drug exposure during pregnancy (Standardised MedDRA Queries pregnancy and neonatal topics [broad]). Effectiveness endpoints were the time course of arterial blood pH and other poisoning and toxicity parameters and toxicity symptoms; outcomes (survived without sequelae, survived with sequelae, death) of fomepizole treatment were assessed as both safety and effectiveness endpoints.
2.6. Statistical analysis
Safety outcomes were analyzed in the safety analysis set, defined as patients who received fomepizole, had no significant protocol violation, and had information on presence or absence of AEs. Effectiveness outcomes were analyzed in the effectiveness analysis set, defined as patients included in the safety analysis set who met the indication for fomepizole treatment, had no significant protocol violation, and had evaluable effectiveness data. Patients who met all of the following criteria were considered as not having evaluable data: no data available for arterial blood pH at baseline and from at least one time point from 0.5 hours after baseline to up to 24 hours after the completion of the final infusion; no data available for at least one of the poisoning/toxicity test parameters at baseline and from at least one time point from 0.5 hours after baseline to up to 24 hours after the completion of the final infusion; no data available for at least one toxic symptom parameter at baseline or at the completion of the final infusion; and no documented outcome. Safety outcomes, including AEs and ADRs, were reported as n (%), and the time course of arterial blood pH and poisoning/toxicity test parameters were reported as mean, standard deviation (SD), median, minimum, maximum, and interquartile range. Absolute change from baseline in arterial blood pH and poisoning/toxicity test parameters were summarized. The presence and absence of toxic symptoms at baseline were cross-tabulated against the presence and absence of toxic symptoms at the completion of final infusion; patients with each outcome at hospital discharge or at time of transfer to another department were reported as n (%). Furthermore, details of any sequelae were also summarized descriptively. AEs and ADRs were coded by PT and SOC using the Japanese translation of MedDRA (MedDRA/J) Version 25.0. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient disposition
Of the 147 registered patients across 91 medical institutions, case report forms were collected for 137 patients (). Of those, 131 patients were included in the safety analysis set after excluding six patients whose presence or absence of AEs could not be determined (i.e. recorded as ‘unknown’ in the case report form). The effectiveness analysis set included a total of 126 patients, excluding five patients who received fomepizole treatment outside of its indication for use.
3.2. Demographic and baseline clinical characteristics
Of the 131 patients included in the safety analysis set, 87 (66.4%) patients were male, mean (SD) age was 43.6 (20.7) years, and about half (49.6%) of the patients were diagnosed with methanol poisoning (). The most common route of ingestion of poison was oral (89.3%), and the most common reason for ingestion was suicide attempt (67.2%). Of the 97 patients with available data, mean (SD) time to receive fomepizole from the time of ingesting the poison was 15.1 (11.5) hours, with almost half (45.4%) of patients receiving fomepizole in less than 12 hours of ingesting the poison.
Table 1. Patient demographics and clinical characteristics (safety analysis set).
Acute blood purification therapy (hemodialysis) was received concomitantly with fomepizole by 87 (66.4%) patients (). Of those, 12 (13.8%) patients received both continuous and intermittent hemodialysis; 30 (34.5%) patients received continuous hemodialysis and 45 (51.7%) patients received intermittent hemodialysis. Other non-pharmacological procedures/interventions were artificial ventilation in 49 (37.4%) patients and gastrointestinal decontamination (e.g. gastric lavage) in 37 (28.2%) patients. Furthermore, concomitant medications were received by 124 (94.7%) patients. The most common medication was sodium bicarbonate (23.4%), followed by folic acid (17.7%). Mean (SD) number of concomitant medications received was 4.1 (3.1).
Table 2. Concomitant non-pharmacological procedures/interventions and medication (safety analysis set).
3.3. Fomepizole treatment
Among the patients included in the safety analysis set, mean (SD) number of days for which the patients received fomepizole was 2.4 (1.7) days. Mean (SD) initial and cumulative fomepizole dose was 901.6 (275.3) mg and 3253.5 (3305.2) mg; 84.7% of the patients (111/131) received fomepizole at a dose of 15 mg/kg and mean (SD) maximum number of doses in which the patients received the treatment was 4.5 (4.2). More than 65% of the patients received fomepizole over a period from 30 to less than 60 minutes. The majority (87.0%) of patients discontinued fomepizole because the treatment goal was achieved, as determined by the investigator (multiple answers allowed). Other reasons for discontinuation were the occurrence of AEs (2.3%), insufficient effect (1.5%), and other (11.5%).
3.4. Safety
In the safety analysis set, 37 AEs occurred in 22 (16.8%) patients. A total of 16 SAEs occurred in 13 (9.9%) patients, which included death and chemical poisoning (two patients each: 1.5%). No SAEs were considered to be related to fomepizole, and thus no serious ADRs were reported (). Eleven ADRs were reported in seven (5.3%) patients (). The most common ADR was vomiting, reported in three (2.3%) patients. No ADRs identified in the safety specifications of anaphylactic reaction and central nervous system disorder were reported. Furthermore, no patients in the surveillance population were reported to be pregnant, and therefore no patients reported fetal disorder due to drug exposure during pregnancy.
Table 3. Summary of ADRs and serious ADRsa (safety analysis set).
Most patients (more than 80%) survived without any sequelae in both the safety analysis set and the effectiveness analysis set (). Survival with sequelae was reported in 19 (14.5%) patients in the safety analysis set and 18 (14.3%) in the effectiveness analysis set. Most frequently reported sequelae were renal failure or renal dysfunction (safety analysis set: nine patients; effectiveness analysis set: eight patients), disorders of consciousness (safety analysis set: three patients; effectiveness analysis set: three patients), and visual impairment (safety analysis set: two patients; effectiveness analysis set: two patients). Death was reported in seven (5.3%) and five (4.0%) patients in the safety analysis set and the effectiveness analysis set, respectively. None of the sequelae or deaths reported were considered to be related to fomepizole treatment by the investigators at each study site.
Table 4. Summary of outcomes.
3.5. Effectiveness
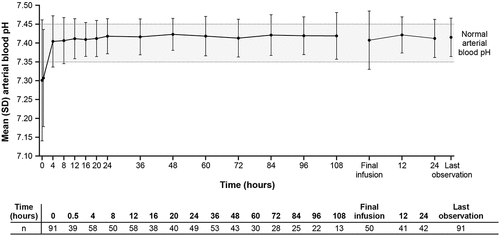
Mean (SD) arterial blood pH at baseline was 7.301 (0.1606); this increased and reached 7.404 (0.0681) by 4 hours after baseline (); the pH level remained around the normal blood pH level of 7.35–7.45 [Citation22] through 108 hours after baseline, at 12 and 24 hours after the completion of the final infusion, and at last observation.
Figure 2. Arterial blood pH over time. Dotted line represents normal blood pH (effectiveness analysis set, N = 126). Shaded area represents the normal range of arterial blood pH.
Mean (SD) PaCO2 increased from 32.18 (10.718) mmHg at baseline to 35.30 (6.723) mmHg at 4 hours after baseline (Supplemental Figure S1a); this remained stable through 108 hours after baseline, at 12 and 24 hours after the completion of the final infusion, and at the last observation. Mean (SD) PaO2 steeply decreased from baseline to 8 hours after baseline, which then continued to decrease slowly, reaching the upper range of the normal PaO2 (100 mmHg) [Citation22] at approximately 24 hours after baseline, and remained stable through 108 hours after baseline (Supplemental Figure S1b). PaO2 levels remained between the normal PaO2 level at 12 and 24 hours after the final infusion and at last observation. Mean (SD) bicarbonate ion level increased steeply from baseline to 4 hours after baseline, reaching the lower range of the normal bicarbonate ion level of around 22 mEq/L [Citation22] (Supplemental Figure S1c); this continued to increase slowly up to 20 hours after baseline, then plateaued from 20 hours through 108 hours after baseline, at 12 and 24 hours after the completion of the final infusion, and at last observation. Mean (SD) BE level increased from −9.09 (9.170) mEq/L at baseline to −2.19 (4.586) mEq/L at 4 hours after baseline, then stabilized around 1–2 mEq/L from 20 hours after baseline through to the final infusion, at 12 and 24 hours after the completion of the final infusion, and at last observation (Supplemental Figure S1d). Mean (SD) anion gap at baseline was 17.40 (8.858) mEq/L but decreased below 12 mEg/L by 4 hours after baseline, plateauing around 6.5–8.5 mEq/L from 12 hours after baseline through to the final infusion, at 12 and 24 hours after the completion of the final infusion, and at last observation (Supplemental Figure S1e).
The proportion of patients who presented with toxic symptoms decreased at the completion of the final infusion relative to baseline for most toxic symptoms, including tachypnea (22.2% to 2.4%), visual impairment (4.0% to 2.4%), disturbance of consciousness (21.4% to 15.9%), metabolic acidosis (laboratory findings; 42.9% to 5.6%), and osmolar gap abnormality (23.8% to 10.3%) (Supplemental Table S1). Conversely, the proportion of patients presenting with the following toxic symptoms increased at the completion of the final infusion relative to baseline: renal failure (4.0% to 12.7%), abnormal renal function test (1.6% to 13.5%), abnormal liver function test (4.0% to 11.9%), and abnormalities in some electrolytes (sodium, calcium, and phosphorus).
4. Discussion
This is the first PMS study that evaluated the safety and effectiveness of fomepizole in patients with ethylene glycol and methanol poisoning in clinical practice in Japan. In this study, fomepizole was well tolerated with no new safety concerns identified. The incidence of ADRs was low, with the majority of patients surviving without any sequelae; no serious ADRs or safety specification of anaphylactic reaction and central nervous system disorder were observed. Furthermore, fomepizole improved poisoning and toxicity test results. Particularly, arterial blood pH improved to the normal level by around 4 hours post-infusion and remained at the normal level even at 24 hours after the last infusion. These results support the use of fomepizole for the treatment of patients with ethylene glycol and methanol poisoning in Japan.
ADRs listed in the Japanese package insert include anaphylaxis (incidence unknown), headache, and injection site reaction (at least 5%) [Citation21]; anaphylaxis and central nervous system disorders are also listed as important identified and potential risks in the Japanese risk management plan [Citation23]. In this study, although serious AEs, which included death, were reported in 16 (9.9%) patients, none of these events were considered to be related to fomepizole and classified as serious ADRs. No anaphylaxis or central nervous system disorders were reported. Moreover, the ADRs identified in this study are consistent with the current precautions for fomepizole, with no new safety concerns identified during the study. The incidence of ADRs was also low (5.3%) and was lower than that reported in previous non-Japanese studies [Citation13,Citation14,Citation16]. In a multicenter prospective study of 19 patients with ethylene glycol poisoning, 31.6% (6/19) of patients reported adverse effects possibly related to fomepizole (bradycardia, seizure, and headache in two patients each) [Citation13]. A similar prospective study of 11 patients with methanol poisoning reported 54.5% (6/11) of patients with AEs classified as possibly related to fomepizole by the treating physician [Citation14]. Furthermore, in a 16-year post-marketing study conducted in France, ADRs were reported in 7% (36/536) of patients and were all considered mild and transient [Citation16]. The numerically lower ADR rates observed in the current study may be explained by the ethnic differences (Japanese patients vs non-Japanese patients from the United States and France) and the longer study duration (7 years in this study vs 16 years in the French post-marketing study). These results suggested that fomepizole is well tolerated when used for the treatment of ethylene glycol and methanol poisoning.
Patients with ethylene glycol or methanol poisoning can develop central nervous system depression, cardiopulmonary and renal failure (ethylene glycol), visual impairment, cardiovascular shock, and respiratory failure (methanol) [Citation6,Citation7]. These symptoms can progress to serious sequelae, including permanent blindness and kidney damage, or death, with delayed treatment [Citation5,Citation24]. To date, there are limited reports on the prognosis of ethylene glycol or methanol poisoning, particularly in Japanese patients. In previous non-Japanese studies, the mortality rate ranged from 0.3% to 27.8% with ethylene glycol poisoning [Citation13,Citation25–28], and from 0% to 44.4% with methanol poisoning [Citation1,Citation29–33]. Particularly, in two previous clinical trials of fomepizole, the mortality rate was 5.3% (1/19 patients) and 18.2% (2/11 patients) in patients with ethylene glycol and methanol poisoning, respectively [Citation13,Citation14]. Moreover, the proportion of patients with and without sequelae after ethylene glycol or methanol poisoning ranged from 10% to 40% and 36% to 73%, respectively [Citation1,Citation14,Citation28–31,Citation33]. As not all patients in these previous studies were treated with fomepizole, a direct comparison is not possible; however, the present PMS study generally reported a lower mortality rate (approximately 5%) and a higher proportion of patients who survived without sequelae (approximately 80%) compared with those previous studies, which may again be explained by the differences in study designs and patient and clinical characteristics between the current study and previous studies. Furthermore, in this study, although approximately 15% of the patients had survived with sequelae, including renal failure or impairment and visual impairment, these were not considered to be related to fomepizole treatment and could possibly be related to the underlying disease and/or the prognosis of the poisoning itself. Fomepizole works by blocking the formation of toxic metabolites of ethylene glycol and methanol [Citation7] and is thought to be most effective when initiated in the early phase of the intoxication [Citation7], preventing ethylene glycol-related renal failure and methanol-related visual and neurological injuries [Citation5]. Time to treatment initiation influences mortality associated with ethylene glycol and methanol poisoning [Citation8]. Previous studies have also reported that the degree of metabolic acidosis is correlated with poor prognosis [Citation29]. In this study, almost half of patients started fomepizole in less than 12 hours, with approximately 83% of the patients starting treatment in less than 24 hours of ingestion of these alcohols. Early treatment with fomepizole may have blocked the formation of toxic metabolites and lessened the degree of metabolic acidosis, contributing to the high rate of survival without sequelae and the low mortality rate reported in the current study. Therefore, these results again provide valuable evidence on the safety of fomepizole treatment, as well as its effectiveness in improving the prognosis of patients with ethylene glycol and methanol poisoning in Japan, and suggest the importance of initiating treatment as early as possible.
Metabolic acidosis presents as a result of glycolate and formate accumulation during ethylene glycol and methanol metabolism, respectively [Citation5]. Following metabolic acidosis, patients develop hyperventilation to compensate for the decrease in blood pH [Citation5]. In a previous study of patients with ethylene glycol poisoning, arterial pH of 7.24 at baseline progressively increased to approximately 7.35 by about 4 hours of fomepizole treatment [Citation13]. Similarly, in this study, mean arterial blood pH of 7.3 at baseline returned to a normal blood pH of around 7.4 [Citation22] by approximately 4 hours of starting fomepizole treatment and remained stable even after treatment completion. Corresponding to the improvement in arterial blood pH, improvement in PaCO2, PaO2, bicarbonate ion level, and BE were also observed in this study. Moreover, most toxic symptoms observed at baseline appeared to have resolved at the end of final fomepizole infusion. These results suggest that fomepizole improves arterial blood pH and other toxicity parameters, demonstrating results consistent with those previously reported of fomepizole for the treatment of ethylene glycol or methanol poisoning. These effectiveness results, together with the safety results observed in the current study, provide valuable and comprehensive information that supports the use of fomepizole in patients with ethylene glycol and methanol poisoning.
This PMS study was the first study in Japan to include patients from all institutions that prescribed fomepizole and agreed to participate in the study. To date, data on a small number of patients from case studies only are available in Japan; therefore, the current study provides valuable real-world data on the safety and effectiveness of fomepizole in a larger number of patients (>100 patients) with ethylene glycol and methanol poisoning in Japan. Additionally, this study evaluated the safety and effectiveness of fomepizole treatment using various outcome measures, including arterial blood pH, and other toxicity parameters and toxic symptoms. However, this study was limited by its retrospective nature. It was difficult to assess the effect of fomepizole alone, and the study results may have been affected by the presence of potential confounding factors, such as baseline characteristics, and the variation in dosage of fomepizole actually administered among the patients. Although fomepizole was used concomitantly with non-pharmacological procedures/interventions and medications, including hemodialysis (66.4%), bicarbonate (23.4%), and ethanol (3.2%), no subanalyses were conducted to evaluate the effect of these different variables. Furthermore, no sensitivity analyses were conducted. A paired t-test was planned and performed to test for statistical significance of the change from baseline in arterial blood pH and poisoning/toxicity test parameters. However, the test results are not shown because the test did not adjust for multiplicity and were for reference only. Data on the various toxicity parameters between the start of fomepizole treatment and hemodialysis were also not available.
5. Conclusions
In this PMS study, fomepizole demonstrated a high survival rate without sequelae; no patients were found to have worsening of toxicity symptoms or test results. These results suggest that fomepizole may contribute to the improvement of clinical outcomes and prognosis in patients with ethylene glycol and methanol poisoning in Japan.
Declaration of interest
Momoha Koyanagi and Naoki Yoshida are employees of Takeda Pharmaceutical Company Limited. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution statement
All authors participated in the interpretation of study results, and in the drafting, critical review, and approval of the final version of the manuscript. TY was involved in the study design, and MK and NY were involved in the study design, data collection, and analysis. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download PDF (174.8 KB)Acknowledgments
The authors would like to thank all study participants, and also contributors within Takeda Pharmaceutical Company Limited for their cooperation in conducting the study and preparing the manuscript. Medical writing assistance was provided by Hana Nomura, BPharm (Hons), CMPP, and Prudence Stanford, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by Takeda Pharmaceutical Company Limited. ProScribe’s services complied with international guidelines for Good Publication Practice. The study was registered at the Japanese Pharmaceutical Information Center (identifier: JapicCTI-152817) and at ClinicalTrials.gov (identifier: NCT02415712).
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this manuscript, will be made available within 3 months from the initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with the applicable privacy laws, data protection, and requirements for consent and anonymization.
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2024.2372410
Additional information
Funding
References
- Zakharov S, Navratil T, Pelclova D. Fomepizole in the treatment of acute methanol poisonings: experience from the Czech mass methanol outbreak 2012-2013. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(4):641–649. doi: 10.5507/bp.2014.056
- McQuade DJ, Dargan PI, Wood DM. Challenges in the diagnosis of ethylene glycol poisoning. Ann Clin Biochem. 2014;51(Pt 2):167–178. doi: 10.1177/0004563213506697
- Nekoukar Z, Zakariaei Z, Taghizadeh F, et al. Methanol poisoning as a new world challenge: a review. Ann Med Surg (Lond). 2021;66:102445. doi: 10.1016/j.amsu.2021.102445
- Patel R, Mistry AM, Mistry CM. Unintentional ethylene glycol poisoning in an adolescent. Cureus. 2020;12(11):e11521. doi: 10.7759/cureus.11521
- Mégarbane B. Treatment of patients with ethylene glycol or methanol poisoning: focus on fomepizole. Open Access Emerg Med. 2010;2:67–75. doi: 10.2147/OAEM.S5346
- McMartin K, Jacobsen D, Hovda KE. Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol. 2016;81(3):505–515. doi: 10.1111/bcp.12824
- Rietjens SJ, de Lange DW, Meulenbelt J. Ethylene glycol or methanol intoxication: which antidote should be used, fomepizole or ethanol? Neth J Med. 2014;72(2):73–79.
- Gallagher N, Edwards FJ. The diagnosis and management of toxic alcohol poisoning in the emergency department: a review article. Adv J Emerg Med. 2019;3(3):e28. doi: 10.22114/ajem.v0i0.153
- Hoyte C, Schimmel J, Hadianfar A, et al. Toxic alcohol poisoning characteristics and treatments from 2000 to 2017 at a United States regional poison center. DARU, J Pharm Sci. 2021;29(2):367–376. doi: 10.1007/s40199-021-00418-4
- Askarian M, Khakpour M, Taghrir MH, et al. Investigating the epidemiology of methanol poisoning outbreaks: a scoping review protocol. JBI Evid Synth. 2021;19(6):1388–1393. doi: 10.11124/JBIES-20-00221
- Yousefinejad V, Moradi B, Mohammadi Baneh A, et al. Prognostic factors of outcome in methanol poisoning; an 8-year retrospective cross-sectional study. Arch Acad Emerg Med. 2020;8(1):e69.
- McMartin K, Brent J. Analysis of fomepizole elimination in methanol- and ethylene glycol-poisoned patients. J Med Toxicol. 2022;18(1):19–29. doi: 10.1007/s13181-021-00862-3
- Brent J, McMartin K, Phillips S, et al. Fomepizole for the treatment of ethylene glycol poisoning. Methylpyrazole for toxic alcohols study group. N Engl J Med. 1999;340(11):832–838. doi: 10.1056/NEJM199903183401102
- Brent J, McMartin K, Phillips S, et al. Fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344(6):424–429. doi: 10.1056/NEJM200102083440605
- Nakhaee S, Naseri K, Mehrpour O. Toxic alcohol diagnosis and management: an emergency medicine review-comment. Intern Emerg Med. 2019;14(7):1183–1184. doi: 10.1007/s11739-018-02012-0
- Rasamison R, Besson H, Berleur MP, et al. Analysis of fomepizole safety based on a 16-year post-marketing experience in France. Clin Toxicol (Phila). 2020;58(7):742–747. doi: 10.1080/15563650.2019.1676899
- Amano K, Morita M, Ebihara T, et al. Fomepizole for ethylene glycol poisoning: two case reviews. J Jpn Assoc For Acute Med. 2018;29(1):12–17. Japanese.
- Nosaka H, Keiji S. A case of severe ethylene glycol poisoning that was successfully treated with early administration of fomepizole and continuous renal replacement therapy. J Jpn Soc For Dialysis Ther. 2019;52(5):291–293. Japanese.
- Yoshida S, Nakano K, Inoue Y, et al. Methanol poisoning diagnosed by emergency toxicological analysis and treated with fomepizole and hemodialysis: a case report. Jpn J Clin Toxicol. 2019;32(4):410–414. Japanese.
- Ono Y, Hata N, Sonoda Y, et al. Fomepizole alone therapy for a case of methanol intoxication. J Jpn Assoc For Acute Med. 2017;28(7):275–280. Japanese.
- Takeda Pharmaceutical Company Limited. Package insert, Fomepizole intravenous infusion 1.5g “Takeda”. 2023 [cited 2023 Nov 30]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/400256_3929411A1020_1_05. Japanese.
- Singh V, Khatana S, Gupta P. Blood gas analysis for bedside diagnosis. Natl J Maxillofac Surg. 2013;4(2):136–141. doi: 10.4103/0975-5950.127641
- Takeda Pharmaceutical Company Limited. Drug risk management plan, Fomepizole for intravenous infusion 1.5g. 2023 [cited 2023 Nov 30]. Available from: https://www.pmda.go.jp/RMP/www/400256/32827bba-97e4-4ac5-ae0b-f205e36f3d30/400256_3929411A1020_001RMP.pdf. Japanese.
- Zimmerman HE, Burkhart KK, Donovan JW. Ethylene glycol and methanol poisoning: diagnosis and treatment. J Emerg Nurs. 1999;25(2):116–120. doi: 10.1016/S0099-1767(99)70156-X
- Karlson-Stiber C, Persson H. Ethylene glycol poisoning: experiences from an epidemic in Sweden. J Toxicol Clin Toxicol. 1992;30(4):565–574. doi: 10.3109/15563659209017942
- Tanasescu A, Macovei RA, Tudosie MS. Outcome of patients in acute poisoning with ethylene glycol–factors which may have influence on evolution. J Med Life. 2014;7 Spec No.3(Spec Iss 3):81–86.
- Jobson MA, Hogan SL, Maxwell CS, et al. Clinical features of reported ethylene glycol exposures in the United States. PLoS One. 2015;10(11):e0143044. doi: 10.1371/journal.pone.0143044
- Latus J, Kimmel M, Alscher MD, et al. Ethylene glycol poisoning: a rare but life-threatening cause of metabolic acidosis-a single-centre experience. Clin Kidney J. 2012;5(2):120–123. doi: 10.1093/ckj/sfs009
- Hovda KE, Hunderi OH, Tafjord AB, et al. Methanol outbreak in Norway 2002-2004: epidemiology, clinical features and prognostic signs. J. Intern. Med. 2005;258(2):181–190. doi: 10.1111/j.1365-2796.2005.01521.x
- Massoumi G, Saberi K, Eizadi-Mood N, et al. Methanol poisoning in Iran, from 2000 to 2009. Drug Chem Toxicol. 2012;35(3):330–333. doi: 10.3109/01480545.2011.619193
- Paasma R, Hovda KE, Tikkerberi A, et al. Methanol mass poisoning in Estonia: outbreak in 154 patients. Clin Toxicol (Phila). 2007;45(2):152–157. doi: 10.1080/15563650600956329
- Kane RL, Talbert W, Harlan J, et al. A methanol poisoning outbreak in Kentucky. A clinical epidemiologic study. Arch Environ Health. 1968;17(1):119–129. doi: 10.1080/00039896.1968.10665199
- Mégarbane B, Borron SW, Trout H, et al. Treatment of acute methanol poisoning with fomepizole. Intensive care Med. 2001;27(8):1370–1378. doi: 10.1007/s001340101011