Abstract
Some 6-fluoro-5-substituted-benzimidazole derivatives in which indole and 1,1,4,4-tetramethyl-1,2,3,4-tetrahydro-naphthalene groups were attached to the 2-position of the benzimidazole ring were synthesized and tested for antioxidant properties in vitro. Almost all the synthesized compounds at the 10− 3 M concentrations showed superoxide anion scavenging activity. Compounds 5, 3, 9, 4, 17 and 13 have strong inhibitory effects on superoxide anion formation (98%, 93%, 91%, 88%, 85% and 81%, respectively) at 10− 3 M concentration and these results are better than 30 IU of superoxide dismutase (SOD) (76%). Compound 11 is the most effective scavenger of 2,2-diphenyl-1-picrylhydrazyl (DPPH) stable free radical at 10− 3 M (61%) concentration.
1 Introduction
Free radicals interfere with many diseases including inflammation, atherosclerosis, shock, ischemic disease, and cancerCitation1-3. Hydroxyl (OH√), superoxide anion nitric oxide (NO√) and peroxyl
radicals, reactive oxygen species (ROS), are involved in different physiological processes[Citation4,Citation5]. Protection of biological molecules such as lipids, carbohydrates, proteins and DNA from oxidative stress is very important to prevent inflammatory diseases, such as atherosclerosis, aging and cancer, accentuated by high levels of ROS[Citation6]. Antioxidants scavenge and prevent the formation of free radicals so they are highly important for the potential treatment of these kinds of diseases, so that in recent years, there has been an increasing interest in finding new antioxidant compounds.
Melatonin (N-acetyl-5-methoxytryptamine) () is a free radical scavenger and antioxidant. Due to the stimulation of several antioxidative enzymes i.e., SOD, glutathione peroxidase (GPx), and glutathione reductase (GRd), melatonin increases antioxidant effectiveness of these enzymes[Citation7]. All trans-retinoic acid (ATRA) and retinol (vitamin A) (), which are natural retinoids, are used clinically for the treatment of proliferative dermatological diseases and for the prevention of some tumors[Citation8]. A number of retinoic acid derivatives, termed retinoids as a biological general term[Citation9], have been reported and their antioxidant potencies have been investigatedCitation10-12. However, some benzimidazole retinoids have been reported as retinoid antagonists[Citation13].
Recently, many reports from us and others have revealed the antioxidant properties of some novel indole and benzimidazole derivativesCitation10-12Citation14–16. In connection with these investigations our studies continue to search for new benzimidazole derivatives having potent antioxidant activities.
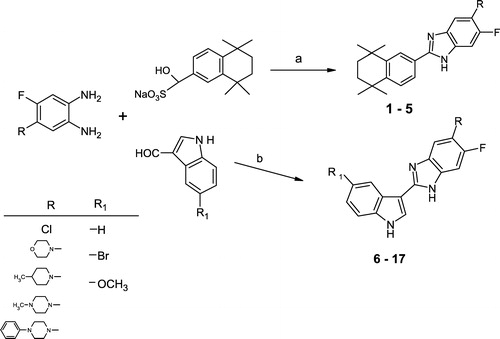
In this study we have aimed to connect both indole and tetrahydronaphthalene fragments to the benzimidazole ring. Synthesis of some new 5-substituted-6-fluoro-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)-1H-benzimidazoles (1-5) and 5-substituted-6-fluoro-2-(5-substituted-1H-indol-3-yl)-1H-benzimidazole derivatives (6-17) was performed and their free radical scavenging properties examined in vitro by determining their capacity to scavenge superoxide anion formation and to interact with the stable free radical DPPH.
2 Materials and methods
2.1 Chemistry
Uncorrected melting points were measured with an Electrotermal melting point apparatus. 1H NMR spectra were recorded on a Varian Mercury 400 MHz spectrometer in DMSO-d6, Chemical shifts are expressed as δ (ppm) values with tetramethylsilane (TMS) as an internal standard and coupling constants (J) are reported in Hertz and multiplicity as s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and br = broad peak. Mass spectra were determined on a Waters Micromass ZQ Ms spectrometer using the ESI technique. Elemental analyses (C, H, N) were determined on a Leco CHNS 932 instrument (St.Joseph, M1 USA), and were within ± 0.4% of the theoretical values. All instrumental analyses were performed at Ankara University, Faculty of Pharmacy. Analytical thin-layer chromatography (TLC) was run on Merck silica gel plates (Kieselgel 60F-254). Column chromatography was conducted on silica gel 60 (40–63 μm particle size) (Merck). All starting materials and reagents were high-grade commercial products purchased from Aldrich, Merck or Fluka.
For the synthesis of the final compounds (1-17) the reaction sequences are outlined in and the substituents of 1-17 are shown in . The target compounds (1-17) were synthesized via condensation of related o-phenylenediamines with the sodium metabisulfite adduct of 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalene-2-carbaldehyde in DMF (Method a) or with substituted indole and sodium metabisulfite in ethanol (method b). o-Phenylenediamines,[Citation17,Citation18] substituted indole carboxyaldehydes[Citation19] and 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalene-2-carbaldehyde sodium metabisulfite salt,[Citation10,Citation11] which are all required in this study, were synthesized by a few steps according to published procedures.
Figure 2 Synthetic scheme for the preparation of 5-substituted-6-fluoro-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)-1H-benzimidazole derivatives (1-5) and 5-substituted-6-fluoro-2-(5-substituted-1H-indol-3-yl)-1H-benzimidazole derivatives (6-17). see for R, R′-substituents for respective compounds.
The structures of the synthesized compounds were consistent with their 1H NMR spectra, and molecular weights of these compounds were determined by ESI mass spectra. Some physical and spectral data for 1-17 are summarized in .
Table I. Physical and spectral data for compounds 1-17.
2.1.1 General procedure for the preparation of 5-substituted-6-fluoro-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)-1H-benzimidazole derivatives (1-5)
A solution of 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalene-2-carbaldehyde (6.6 g, 30 mmol) in 20 mL EtOH was added to a solution of Na2S2O5 (3.12 g, 30 mmol) in 20 mL water and the mixture stirred in an ice-bath to give a white precipitate which was filtered and dried (m.p. decom. >300°C). The mixture of the sodium metabisulfite adduct of 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalene-2-carbaldehyde (2 mmol) and the appropriate 1,2-phenylenediamine (2 mmol) in DMF (5 mL) were heated at 130°C for 4 h. The reaction mixture was cooled, poured into the water, and the solid was filtered. The purification procedure and some spectral data for the synthesized compounds are given in .
2.1.2 General procedure for the preparation of 5-substituted-6-fluoro-2-(5-substituted-1H-indol-3-yl)-1H-benzimidazole derivatives (6-17)
A mixture of the appropriate o-phenylendiamine (1 mmol), related indole derivative (1 mmol) and Na2S2O5 (40%) (2 mL) in EtOH (4 mL), was refluxed under nitrogen atmosphere for 4 h. The reaction mixture was poured into water, and the precipitate was filtered and washed with water. The purification procedure and some spectral data for the synthesized compounds are given in .
2.2 Biological activity studies
2.2.1 Superoxide radical scavenging activity
The capacity of the benzimidazole compounds 1-17 to scavenge superoxide anion formation was determined spectrophotometrically on the basis of inhibition of cytochrome c reductase according to the modified method of McCord et al[Citation20]. The results are shown in . Superoxide anion was generated in the xanthine/xanthine oxidase system. The reaction mixture contained in a final volume of 1 mL, consisted of 0.05M phosphate buffer pH 7.8, 0.32 U xanthine oxidase, 50 μM xanthine, 60 mM ctytochrome c and different concentrations of synthesized compounds in 100 μL of DMSO. DMSO was used as the control solution. The absorbance was measured spectrophotometrically at 550 nm for cytochrome c reduction. Each experiment was performed in triplicate, and the results are expressed as a percentage of the control value.
Table II. Structures and effects of the compounds (1-17) on DPPH free radical and superoxide anion radical scavenging activity.a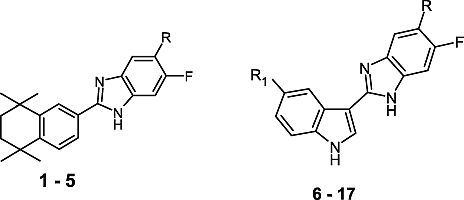
2.2.2 DPPH free radical scavenging activity
The free radical scavenging activities of compounds (1-17) were tested by their ability to bleach the stable radical DPPH[Citation21]. The results are shown in . This assay has often been used to estimate the antiradical activity of antioxidants. Because of its odd electron, DPPH gives a strong absorption band at 517 nm in visible spectroscopy. The reaction mixture contained 100μM DPPH in methanol and different concentrations of compounds. Absorbance at 517 nm was determined after 30 min at room temperature and the scavenging activity was calculated as a percentage of radical reduction. Each experiment was performed in triplicate. DMSO was used as a control solution and BHT as a reference compound.
2.2.3 Log P
Hydrophobicity, expressed as log P values, of compounds synthesized in this study was calculated with the programme CS Chem Draw Pro 5.0 CambridgeSoft Corporation, based on Crippen's fragmentation, Viswanadhan's fragmentation and Broto's method[Citation22,Citation23].
3 Results and discussion
Free radical scavenging properties of tetrahydronaphthalene (1-5) and indolobenzimidazole (6-17) derivatives were examined by determining their scavenging capability for superoxide anion and their interaction with the stable free radical DPPH.
In the present study our results on superoxide radical scavenging and DPPH free radical scavenging activity did not correlate. The compounds showed greater scavenger activity against superoxide radical than against DPPH free radical.
The results of the inhibitory effects of different concentrations of synthesized compounds on superoxide anion showed that almost all the synthesized compounds at 10− 3 M concentration had superoxide radical scavenging activity, the scavenging extent being in the range 22–98%. Compounds 5, 3, 9, 4, 17 and 13 having methylpiperazine and phenylpiperazine substitutents on the benzimidazole ring had a strong scavenging effect on superoxide anion (98%, 93%, 91%, 88%, 85% and 81%, respectively) at 10− 3 M concentration. Compounds 11, 1 and 6 also showed a scavenging effect on superoxide anion formation by about 67%, 58%, and 55%, respectively, at 10− 3 M concentration. Compound 2 had no effect on superoxide anion formation. Because compound 8 increases the production of superoxide anion at the 10− 3 M concentration but behaves like an antioxidant at the 10− 4 M concentration, it is possible to conclude that it acts like an antioxidant or prooxidant depending on its concentration. Similar examples for this conclusion can be found in reports stating that some antioxidant compounds, i.e. ascorbic acid[Citation24], may behave as an antioxidant or prooxidant depending on the concentration. Compound 7 had no effect on superoxide anion formation in a concentration-dependent manner. Interestingly, compounds 1 and 10 displayed better antioxidant activity at lower concentration (10− 4 M) than at higher concentration (10− 3 M). Compound 10 showed a strong scavenging effect on superoxide anion formation by about 86% at 10− 4 M concentration.
The results in show that the synthesized compounds have no significant effect on DPPH free radical scavenging activity. However, compound 11 had the most effective DPPH scavenging activity at 10− 3 M concentration (61%), followed by compound 13 (53%). For compound 11, a correlation existed between superoxide and DPPH free radical scavenging activities.
Because of the difference in the mechanism of free radical production in oxidative stress and radical scavenging activity of antioxidant compoundsCitation25-27, it was not surprising to observe different effects of the compounds 1-17 on superoxide anion radical formation and DPPH free radical scavenging activity. Such contradictory results have previously been found in the literature[Citation28,Citation29]. In our previous study, we reported that benzimidazole derivatives carrying a triazole ring at the N1 position of benzimidazole were found to interact with the stable free radical DPPH but not to have an effect on superoxide anion radical formation[Citation15].
Lipophilicity has been reported[Citation30,Citation31] to have a positive influence on antioxidant activity so the log P values of 1-17 were calculated, in order to investigate if there was a correlation between lipophilicity and antioxidant activity. There was an interesting correlation between them. Compounds (5, 9, 13 and 17) having a 4-phenylpiperazine-1-yl substitutent at position-5 of the benzimidazole ring had higher lipophilicity and also displayed the best radical scavenging activities. It is well known that the contribution of the tetrahydronaphthalene fragment to lipophilicity proves to be greater than that of the indole ring. Similarly, compounds (1-5) having a retinoid fragment instead of indole had higher log P values and displayed better radical scavenging activity than 6-17. Compound 5 having both tetrahydronaphthalene and 4-phenylpiperazine fragments (Log P = 8.05) displayed the best scavenging effect at 10− 3 M concentration (98%) on superoxide anion radical, a result which is better than that achieved with 30 IU of SOD (76%).
The superoxide anion radical has been implicated in several pathophysiological processes, due to its transformation into more reactive species including hydroxyl radical that initiates lipid peroxidation. Superoxide has also been observed to directly initiate lipid peroxidation[Citation32]. Some herbal plants display antioxidant properties via scavenging of superoxide anion radicalCitation33-35. Therefore, the scavenging of superoxide anion radical by 5, 3, 9, 4, 17 and 13 (98%, 93%, 91%, 88%, 85% and 81%, respectively, at 10− 3 M concentration) is likely to make them promising antioxidants. The results observed here prompt us to further our studies in this way.
References
- McCord JM. N Engl J Med 1986; 312: 159–162
- Olanow CW. Neurology 1990; 40(Suppl. 3)32–37
- Martinez-Cayuela M. Biochimie 1955; 77: 147–161
- Schreck R, Bauerle RA. Trends Cell Biol 1991; 1: 39–42
- Fridovich I. Science 1978; 201: 875–880
- Dong Y, Venkatchalam TK, Narla RK, Trieu VN, Sudbeck EA, Uckun F. Free Rad Biol Med 2000; 10: 87–90
- Karbownik M, Reiter RJ. Proc Soc Exp Biol Med 2000; 225: 9–22
- Chomienne C, Fenaux P, Degos I. FASEB J 1996; 10: 1025–1030
- Sporn MB, Roberts AB, Roche NS, Kagechika H, Shudo K. J Am Acad Derm 1986; 15: 756–764
- Ates-Alagöz Z, Büyükbingöl E. Heterocycl Commun 2001; 7: 455–460
- Ateş Z, Süzen S, Büyükbingöl E, Can-Eke B, İşcan M. Il Farmaco 1997; 52(11)703–706
- Ates-Alagöz Z, Can-Eke B, Çoban T, Iscan M, Buyukbingol E. Arch Pharm Pharm Med Chem 2004; 337: 188–192
- Eyrolles L, Kagechika H, Kawachi E, Fukasawa H, Iijima T, Matsushima Y, Hashimoto Y, Shudo K. J Med Chem 1994; 37: 1508–1571
- Kuş C, Ayhan-Kılcıgil G, Can Eke B, İşcan M. Arch Pharm Res 2004; 27: 156–163
- Ayhan-Kılcıgil G, Kuş C, Çoban T, Can-Eke B, Işcan M. J Enz Inhib Med Chem 2004; 19(2)129–135
- Can-Eke B, Puskullu MO, Buyukbıngol E, Iscan M. Chemico-Biol Interact 1998; 113: 65–67
- Kuş C, Göker H, Altanlar N. Arch Pharm Pharm Med Chem 2001; 334: 361–365
- Sanna P, Carta A, Loriga M, Zanetti S, Sechi L. Il Farmaco 1998; 53: 455–461
- Büyükbingöl E, Süzen S, Klopman G. Il Farmaco 1994; 49(6)443–447
- McCord J, Fridowich I. J Biol Chem 1969; 243: 6049–6055
- Blois MS. Nature 1958; 181: 1199–1200
- Ghose AK, Crippen GM. J Chem Inf Comput Sci 1987; 27: 21–35
- Viswanadhan VN, Ghose AK, Revankar GR, Robins RK. J Chem Inf Comput Sci 1989; 29: 163–172
- Schaefer DM, Liu Q, Faustman C, Yin MC. J Nutr 1995; 125: 1792–1798
- Kombrust DJ, Mavis RD. Mol Pharmacol 1980; 17: 408–414
- Parke DV, Ionnides C, Lewis DF. Can J Physiol Pharmacol 1991; 69: 537–549
- Dix TA. J Chem Res Toxicol 1993; 6: 2–18
- Dündar B, Bozdağ-Dündar O, Can-Eke B, Coban T, İscan M, Buyukbingol E. Pharmazie 2002; 57: 438–441
- Ölgen S, Coban T. Arch Pharm Pharm Med Chem 2002; 7: 331–338
- Ohkawa S, Terao S, Terashita Z, Shibouta Y, Nishikawa K. J Med Chem 1991; 34: 267–276
- Xanthopoulou NJ, Kourounakis AP, Spyroudis S, Kourounakis PN. Eur J Med Chem 2003; 38: 621–626
- Halliwell B. Nutrition Rev 1994; 52: 253–265
- Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Poel BV, Pieters L, Vlietinck AJ, Berghe DV. J Nat Prod 1998; 61: 71–76
- Magnani L, Gaydou EM, Hubaud J-C. Anal Chim Acta 2000; 411: 209–216
- Lee SE, Hwang HJ, Ha J-S, Jeong H-S, Kim JH. Life Sci 2003; 73: 167–179