Abstract
A series of new antibacterial and antifungal coumarin-derived compounds and their transition metal complexes [cobalt (II), copper (II), nickel (II) and zinc (II)] have been synthesized, characterized and screened for their in vitro antibacterial activity against Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Shigella dysenteriae, Bacillus cereus, Corynebacterium diphtheriae, Staphylococcus aureus and Streptococcus pyogenes bacterial strains and for in vitro antifungal activity against Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani, Candida glaberata. The results of these studies show the metal complexes to be more antibacterial and antifungal as compared to the uncomplexed coumarins. The brine shrimp bioassay was also carried out to study their in vitro cytotoxic properties.
Introduction
Coumarins are members of the benzopyrone class, which display not only important structural variety but also significant biological Citation1-5 and pharmacological [Citation6,Citation7] properties. Many of these compounds possess antibacterial [Citation6], antifungal [Citation7] and insecticidal [Citation4] activities. The diverse biological activity of various coumarins as anticoagulants [Citation8], antithrombotics [Citation9], HIV inhibitors [Citation10,Citation11] and human progesterone receptor agonists [Citation12,Citation13] has also been investigated. Several reports on new synthetic routes for these derivatives have been published during the last decade Citation14-20.
Such a variety of interesting biological activity for this class of derivatives prompted us to explore and synthesize some new heteroaromatic- and hydrazide-derived coumarins and investigate their antibacterial and antifungal activity. The ligands, along with their metal complexes were screened for antibacterial activity against Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Shigella dysenteriae, Bacillus cereus, Corynebacterium diphtheriae, Staphylococcus aureus and Streptococcus pyogenes bacterial strains and for in vitro antifungal activity against Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glaberata respectively.
Material and methods
Solvents used were of analytical grade; all metal (II) were used as chloride salts. IR spectra were recorded on a Philips Analytical PU 9800 FTIR spectrophotometer. NMR spectra were recorded on a Perkin-Elmer 283B spectrometer. UV–Visible spectra were obtained in DMF on a Hitachi U-2000 double-beam spectrophotometer. Butterworth Laboratories Ltd carried out the C, H and N analyses. Conductance of the metal complexes was determined in DMF on a Hitachi (Japan) YSI-32 model conduct meter. Magnetic measurements were carried out on solid complexes using Gouy's method. Melting points were recorded on a Gallenkamp (U.K.) apparatus and are not corrected. The complexes were analyzed for their metal content by EDTA titration [Citation21]. Antibacterial, antifungal and cytotoxic screening was done at the HEJ Research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Pakistan.
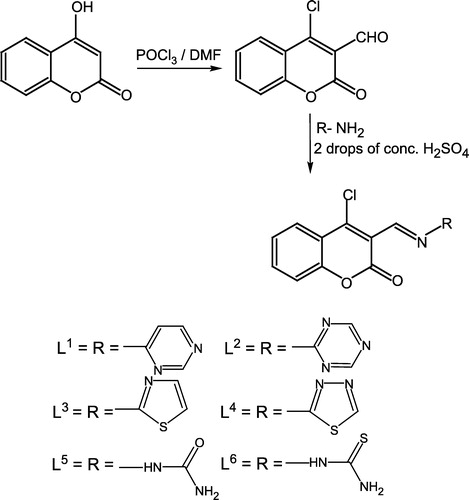
Preparation of ligands (L1–L6) and metal (II) complexes (1–24)
3-Formyl-4-chlorocoumarin
(1) [Citation22] Phosphorus oxychloride (10 mL) was added dropwise to a solution of dimethylformamide (DMF) (20 mL) keeping the temperature below 5oC. solution of 4-hydroxycoumarin (4.0 g) in DMF (10 mL) was then gradually added to the mixture with constant stirring and maintenance of the temperature of the reaction mixture below 5oC. The reaction mixture was then allowed to stand at room temperature for 2 h and then heated on a steam bath for 1 h. After cooling, the reaction mixture was poured onto crushed ice and neutralized with sodium carbonate. A solid product was immediately formed which was crystallized from ethanol to give a yellow solid (80%), m.p. 115oC.
Preparation of ligand (L1)
To a stirred warm ethanolic solution (30 mL) of 4-aminopyrimidine (0.95 g, 0.01 mol) was added 3-formyl-4-chlorocoumarin (1) (1.1 g, 0.01 mol) in ethanol (50 mL). Then 2–3 drops of concentrated H2SO4 were added and the mixture refluxed for 3 h. The completion of reaction was monitored by TLC. After completion the reaction was cooled to afford a solid product. The solid residue was filtered, washed with cold ethanol, then with ether and dried. Crystallization from hot ethanol gave (L1). The same method was applied for the preparation of (L2–L6) by using the corresponding heteroaromatic amines/hydrazides, and working under the same conditions and with the same respective molar ratio.
Preparation of metal (II) complexes (1–24)
For the preparation of metal (II) complexes, a solution (20 mL) of the corresponding ligand (0.02 mol) in hot ethanol was added to a stirred solution of the metal (II) chloride (0.01 mol) in ethanol (25 mL). The mixture was refluxed for 2 h and then cooled to room temperature, when it solidified. The obtained solid was filtered, washed with ethanol, then with ether and dried in air. Crystallization from aqueous/ethanol (30:70) gave the desired metal complex. The same method was used for the preparation of all complexes (1–24) by using the respective metal (II) salts.
Biological activity
Antibacterial bioassay (in-vitro)
All the synthesized ligands (L1–L6) and their corresponding metal (II) complexes (1–24) were screened in-vitro for their antibacterial activity against E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, S. typhi, S. dysenteriae, B. cereus, C. diphtheriae, S. aureus and S. pyogenes using the agar well diffusion method [Citation22]. Two to eight hours old bacterial inoculums containing approximately 104–106 colony forming units (CFU)/ml were used in these assays. The wells were dug in the agar media using a sterile metallic borer with centers at least 24 mm. Recommended concentration (100 μl) of the test sample (1 mg/ml in DMSO) was introduced into the respective wells. Other wells were supplemented with DMSO and the reference antibacterial drug, imipenum, served as negative and positive controls respectively. The plates were incubated immediately at 37oC for 20 h. Activity was determined by measuring the diameter of the zones showing complete inhibition (mm). Growth inhibition was compared with the standard drug. In order to clarify any effect of DMSO or DMF on the biological screening, separate studies were carried out with solutions alone of DMSO and DMF and they showed no activity against any bacterial strains.
Antifungal activity (in-vitro)
Antifungal activities of all compounds were studied against six fungal cultures. Sabouraud dextrose agar (Oxoid, Hampshire, England) was seeded with 105 (cfu) ml− 1 fungal spore suspension and transferred to petri plates. Discs soaked in 20 ml (10 μg/ml in DMSO) of all compounds were placed at different positions on the agar surface. The plates were incubated at 32oC for seven days. The results were recorded as zones of inhibition in mm and compared with the standard drugs miconazole and amphotericin B.
Minimum inhibitory concentration (MIC)
Compounds showing promising antibacterial/antifungal activity were selected for minimum inhibitory concentration studies. The minimum inhibitory concentration was determined using the disc diffusion technique by preparing discs containing 10, 25, 50 and 100 μg/ml of the compounds and applying the reported protocol [Citation23].
Cytotoxicity (in-vitro)
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm) filled with artificial seawater, which was prepared with a commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the minor compartment was open to ordinary light. After two days nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 ml of DMF. From this stock solutions 500, 50 and 5 μg/ml were transferred to 9 vials (three for each dilutions were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 mL of DMF only. The solvent was allowed to evaporate overnight. After two days, when shrimp larvae were ready, 1 mL of seawater and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with seawater to 5 mL per vial. After 24 h the number of survivors was counted. Data were analyzed by a Finney computer program to determine the LD50 values [Citation24].
Results and discussion
Chemistry
The methodology adopted was to first convert 4-hydroxycoumarin into its 4-chloro-3-formyl derivative in the presence of phosphoryl chloride and dimethylformamide (DMF). The aldehyde was then condensed with heterocyclic amines, semicarbazide or thiosemicarbazide leading to a new series of Schiff's bases (Scheme ). The synthesized compounds were further used to prepare their cobalt (II), copper (II), nickel (II) and zinc (II) metal complexes (1–24) which were all characterized by IR, NMR, UV–Visible, molar conductance, magnetic moment and elemental analyses data. The ligands (L1–L6) were prepared by refluxing the appropriate amount of an ethanolic solution of 3-formyl-4-chlorocoumarin with the corresponding heteroaromatic amines/hydrazides, in a 1:1 molar ratio. The structures of these synthesized ligands was established by their IR, NMR and microanalytical data (Tables and ). All metal complexes (1–24) () of these ligands were prepared by the stoichiometric reaction of the corresponding ligand with the respective metal (II) salt as chloride in a molar ratio M:L of 1:2. All the complexes were air and moisture stable and were are intensely colored amorphous solids which decomposed without melting. They were insoluble in common organic solvents and only soluble in water, DMF and DMSO. Molar conductance values of the soluble complexes in DMF (103 M solution at 25oC), indicated high values (86–95 ohm− 1 cm− 2 mol− 1) suggesting that they were all electrolytic in nature [Citation25].
Table I. Physical, spectral and analytical data of the ligands (L1–L6).
Table II. 1H NMR data of the ligands (L1–L6) and their Zn (II) complexes (19–24).
Table III. Physical and analytical data of the metal (II) complexes (1–24).
The elemental analyses data agree well with the proposed formulae for the ligands and metal (II) complexes. Efforts to grow good crystals of the ligands and their metal complexes for X-ray diffraction studies were unsuccessful due to their poor solubility in common organic solvents.
IR spectra
The selected IR spectra of the ligands and their metal complexes along with their tentative assignments are reported in Tables and . The IR spectra of the complexes showed a lower shift of wave numbers in ν(C = N) of the azomethine by 10–20 cm− 1, respectively. The band located at 1715 cm− 1 in all the ligands attributed [Citation26] to ν (C = O) moiety of the coumarin also moved to a lower frequency by 15–20 cm− 1 on coordination. This data on comparison with the spectra of the ligands suggests that the azomethine-N and coumarin-O of the ligands are only involved in coordination with the metal ions. Also, a new band at 315 cm− 1, suggesting [Citation27] coordination of ν(M–Cl) in the Co (II), Ni (II) and Zn (II) complexes was observed while in the spectra of the Cu (II) complexes this band was not observed indicating an octahedral geometry for the Co (II), Ni (II) and Zn (II) complexes and a square-planar geometry for the Cu (II) complexes. The far IR spectra of these metal complexes () exhibited new bands, which are not present in the spectra of the ligands. These bands are located at 435 and 415 cm− 1, and are assigned [Citation28,Citation29] to ν(M–N) of azomethine-N and ν(M = O) of coumarin-O, so supporting evidence for the bonding of the ligands with the metal ions. Accordingly, the above data suggests that the ligands behave as bidentate towards all metals.
NMR spectra
The 1H NMR spectra of the free ligands and their diamagnetic Zn (II) chelates were determined in DMSO-d6. The 1H NMR spectral data are reported along with the possible assignments in . All the protons were found as to be in their expected region [Citation29]. The conclusions drawn from these studies lend further support to the mode of bonding discussed in their IR spectra. In the spectra of the diamagnetic Zn (II) complexes, these protons shifted downfield due to the increased conjugation and coordination to the metal atoms [Citation30]. The number of protons calculated from the integration curves, and those obtained from the values of the expected CHN analyses agreed.
Electronic spectra
The Co(II) complexes exhibited well-resolved, low-energy bands at 7,280–7,375 cm− 1, 17,260–17,385 cm− 1 and a strong high-energy band at 20,485–20,675 cm− 1 () which are assigned [Citation32] to the transitions 4T1g(F) → 4T2g(F), 4T1g(F) → 4A2g(F) and 4T1g(F) → 4T2g(P) for a high-spin octahedral geometry [Citation32]. A high intensity band at 27,175–28,360 cm− 1 was assigned to the metal → ligand charge transfer. The magnetic susceptibility measurements (3.9–4.1 B.M) for the solid Co (II) complexes are also indicative of three unpaired electrons per Co (II) ion suggesting Citation33-41 consistency with their octahedral environment. The electronic spectra of the Cu (II) complexes () showed two low-energy weak bands at 14,710–15,215 cm− 1 and 19,265–19,575 cm− 1 and a strong high-energy band at 30,190–30,285 cm− 1 which was assigned to 2B1g → 2A1g and 2B1g → 2Eg transitions, respectively [Citation42]. The strong high-energy band, in turn, is assigned to metal → ligand charge transfer. Also, the magnetic moment values (1.4–1.6 B.M) () for the copper (II) are indicative of anti-ferromagnetic spin-spin interaction through molecular association [Citation43,Citation44]. The electronic spectra of the Ni (II) complexes showed d-d bands in the region 10,275–10,315, 15,580–15,740 and 26,365–26,555 cm− 1. These are assigned [Citation42] to the transitions 3A2g(F) → 3T2g(F), 3A2g(F) → 3T1g(F) and 3A2g(F) → 3T2g(P), respectively, consistent with their well-defined octahedral configuration. The band at 29,910–30,235 cm− 1 was assigned to metal → ligand charge transfer. The magnetic measurements (3.2–3.4 B.M) showed two unpaired electrons per Ni (II) ion suggesting [Citation44] also an octahedral geometry for the Ni (II) complexes. The electronic spectra of the Zn (II) complexes exhibited only a high-intensity band at 28,275–29,380 cm− 1 and are assigned [Citation42] to a ligand → metal charge transfer.
Table IV. Spectral data of the metal complexes (1–24).
Biological activity
Antibacterial bioassay
All compounds were tested against E. coli, K. pneumoniae, P. mirabilis, P. aeruginosa, S. typhi, S. dysenteriae, B. cereus, C. diphtheriae, S. aureous and S. pyogenes bacterial strains () according to literature protocol [Citation22,Citation23]. The results were compared with those of the standard drug imipenum. All ligands were found potentially active against one or more bacterial strains. Cobalt (II), copper (II), nickel (II) and zinc (II) metal complexes (1–24) of these synthesized ligands (L1–L6) were also screened against the same bacterial strains. It was evident that overall potency of the uncoordinated compounds/ligands was enhanced on coordination with the metal ions.
Table V. In-vitro antibacterial activity data for the ligands (L1–L6) and metal (II) complexes (1–24).
Antifungal bioassay
The antifungal screening of all compounds was carried out against T. longifusus, C. albican, A. flavus, M. canis, F. solani and C. glaberate fungal strains according to the literature protocol [Citation23]. The results were compared with the standard drugs miconazole and amphotericin B. The results given in indicate that all ligands were active against one or more fungal species however, the metal (II) complexes (1–24) of these compounds showed much enhanced activity as compared to the uncoordinated compounds.
Table VI. In-vitro antifungal activity data for ligands (L1–L6) and metal (II) complexes (1–24).
Cytotoxic bioassay
All the synthesized compounds were screened for their cytotoxicity (brine shrimp bioassay) using the protocol of Meyer et al. [Citation38] It was observed that only ligands L5 and L6 and the Cu (II) and Ni (II) metal complexes (11, 12, 17 & 18) displayed a weak cytotoxic activity against Artemia salina, while the other compounds gave values of LD50 (1000 in this assay, and therefore can be considered to be non-cytotoxic.
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) of some selected compounds, which showed significant activity against selected bacterial species, was determined using the disc diffusion method [Citation23]. The MIC of these compounds varies from 10–100 μg/ml. The results shown in indicated that these compounds were the most active in inhibiting the growth of the tested organisms at a 10 μg/ml concentration.
Table VII. Minimum inhibitory concentration (μg/ml) of selected compounds against selected bacteria.
The biological activity of the ligands exhibited a markedly enhancement on coordination with the metal ions against all the test bacterial/fungal strains. The ligands generally showed moderate antibacterial activity against two or four species and insignificant activity against one or two species. However, they showed good antifungal activity against most of the species. It was evident from the data that this activity significantly increased on coordination. This enhancement in the activity of (L1–L6) may be rationalized on the basis that their structures mainly possess an additional C = N bond. It has been suggested that the ligands with nitrogen and oxygen donor systems inhibit enzyme activity, since the enzymes which require these groups for their activity appear to be especially more susceptible to deactivation by metal ions on coordination. Moreover, coordination reduces the polarity [Citation36,Citation37] of the metal ion mainly because of the partial sharing of its positive charge with the donor groups Citation38-43 within the chelate ring system formed during coordination. This process, in turn, increases the lipophilic nature of the central metal atom, which favors its permeation more efficiently through the lipid layer of the micro-organism Citation44-54 thus destroying them more aggressively.
Acknowledgements
The authors (SUR and ZHC) wish to thank the Higher Education Commission, Islamabad (Pakistan) for a research grant to carry out this project.
References
- Murray RDH. Prog Chem Org Nat Prod 1991; 58: 83
- Musajo I, Rodighero G. Experientia 1962; 18: 153
- Montgomery GW, Martin GB, Bars LJ, Pelletier J. J Reprod Fertil 1985; 73: 457
- Naturally occurring insecticides, H Fukami, M Nakijama, M Jacobson, DG Crosby, M Dekker, New York 1971; 71
- Hoult JRS, Payd M. Gen Pharmacol 1996; 27: 713
- Campo AD, Fazzi PL. Riv Ist Steroterap, Ital 1958; 33: 389
- Kumari SS, Rao KRMS, Rao NVS. Proc Ind Acad Sci 1973; 77: 149
- Hossain CF, Okuyama E, Yamazaki M. Chem Pharm Bull 1996; 44: 1535
- Rendenbach-Muller B, Schelcker R, Traut M, Weifenbach H. Bioorg Med Chem Lett 1994; 4: 1195
- Mitra AK, Karchudhurieds ADeN, Misra SK, Mmukhopadhyay AK. J Med Chem 1998; 75: 66
- Zhao H, Nemah N, Hong H, Majumber A, Wang S, Sunder S, Miline GWA, Pommier Y, Bruke TR. J Med Chem 1997; 40: 242
- Romines KR, Morris JK, Howie WJ, Tomich RK, Homg MM, Chong KT, Hinshaw RR, Anderson DJ, Strohbach JW, Turner SR, Mizsak SA. J Med Chem 1996; 39: 4125
- Mohanty N, Rath PC, Rout MK. J Ind Chem Soc 1967; 44: 1001
- Zhi L, Tegley CM, Kallel EA, Marchke KB, Mais DE, Gottardis MM, Jone TK. J Med Chem 1998; 41: 291
- Ghosh CK. J Ind Chem Soc 1991; 68: 21
- Takeehi H, Oda Y, Nishizono N, Oda K, Machida M. Chem Pharm Bull 2000; 48: 1702
- Gennere C, Catlo M, Leonetti F, Weber P, Carrupt PA, Altomane C, Carotti A, Testa B. J Med Chem 2000; 43: 4747
- Main BJ, Tucker H. Prog Med Chem 1985; 22: 21
- Birau MM, Wang ZY. Tetrahedron 2000; 41: 4025
- Heidel ND, Minatelli JA. J Pharm Sci 1981; 70: 84
- Vogel AI. A textbook of quantitative inorganic analysis4th ed. ELBS and Longman, London 1978
- Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR. Vogels' Text book of practical organic chemistry5th ed. Longman (UK) 1994
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. Harwood Academic Publishers, The Netherlands 2001; Vol. 16
- Bauer AW, Kirby WM, Sherris JC, Turck M, Am. J Clin Pathol 1966; 45: 493
- Finney DJ. Probit analysis3rd ed. Cambridge University Press, Cambridge 1971
- Geary WJ. Coord Chem Rev 1971; 7: 81
- Nakamoto K. Infrared spectra of inorganic and coordination compounds2nd ed. Wiley Interscience, New York 1970
- Agarwal RK. J Ind Chem Soc 1988; 65: 448
- Bellamy LJ. Infrared spectra of complex molecules. John Wiley, New York 1971
- Ferrero JR. Low-frequency vibrations of inorganic and coordination compounds. John Wiley, New York 1971
- Simmons WW. The sadtler handbook of proton NMR spectra. Sadtler Research Laboratories, Inc. 1978
- Pasto DJ. Organic structure determination. Prentice Hall International, London 1969
- Lever ABP, Lewis J. J Chem Soc 1963; 2552
- Carlin RL. Transition metal chemistry2nd Ed. Marcel Decker, New York 1965
- Estes WE, Govel DP, Halfield WB, Hodgson DJ. Inorg Chem 1978; 17: 1415
- Balhausen CJ. An introduction to ligand field. McGraw Hill, New York 1962
- Lever ABP. Inorganic electronic spectroscopy. Elsevier, Amsterdam 1984
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Planta Medica 1982; 45: 31
- Chohan ZH, Scozzafava A, Supuran CT. J Enz Inhib Med Chem 2003; 17: 261
- Chohan ZH. Appl Organomet Chem 2002; 16: 17
- Chohan ZH, Farooq MA, Scozzafava A, Supuran CT. J Enz Inhib Med Chem 2002; 17: 1
- Chohan ZH, Rauf A, Supuran CT. Met-Based Drugs 2002; 8: 287
- Chohan ZH, Iqbal MS, Iqbal HS, Scozzafava A, Supuran CT. J Enz Inhib Med Chem 2002; 17: 87
- Hassan MU, Chohan ZH, Supuran CT. Main Group Met Chem 2002; 25: 291
- Chohan ZH, Pervez H, Kausar S, Supuran CT. Synth React Inorg Met-Org Chem 2002; 3: 529
- Chohan ZH, Pervez H, Rauf A, Supuran CT. Met-Based Drugs 2002; 8: 42
- Chohan ZH, Scozzafava A, Supuran CT. J Enz Inhib Med Chem 2003; 18: 259
- Chohan ZH, Supuran CT, Scozzafava A. J Enz Inhib Med Chem 2004; 19: 79
- Chohan ZH, Khan KM, Supuran CT. Appl Organomet Chem 2004; 18: 305
- Chohan ZH. Synth React Inorg Met-Org Chem 2004; 34: 833
- Chohan ZH, Supuran CT, Scozzafava A. J Enz Inhib Med Chem 2004; 19: 79
- Chohan ZH, Pervez H, Khan MK, Rauf A, Supuran CT. J Enz Inhib Med Chem 2004; 19: 51
- Chohan ZH, Pervez H, Khan MK, Rauf A, Supuran CT. J Enz Inhib Med Chem 2004; 19: 85
- Hassan MU, Chohan ZH, Scozzafava A, Supuran CT. J Enz Inhib Med Chem 2004; 19: 263