Abstract
The purification of red blood cell carbonic anhydrase (CA, EC 4.2.1.1) from ostrich (scCA) blood is reported, as well as an inhibition study of this enzyme with a series of aromatic and heterocylic sulfonamides. The ostrich enzyme showed a high activity, comparable to that of the human isozyme II, with kcat of 1.2·106 s− 1 and kcat/KM of 1.8·107 M− 1 s− 1, and an inhibition profile quite different from that of the human red blood cell cytosolic isozymes hCA I and II. scCA has generally a lower affinity for sulfonamide inhibitors as compared to hCA I and II. The only sulfonamide which behaved as a very potent inhibitor of this enzyme was ethoxzolamide (KI = 3.9 nM) whereas acetazolamide and sulfanilamide behaved as weaker inhibitors (inhibition constants in the range 303–570 nM). Several other aromatic and heterocyclic sulfonamides, mostly derivatives of sulfanilamide, homosulfanilamide, 4-aminoethylbenzenesulfonamide or 5-amino-1,3,4-thiadiazole-2-sulfonamide, showed good affinities for the ostrich enzyme, with KI values in the range 25–72 nM.
1 Introduction
The carbonic anhydrases (CAs, EC 4.2.1.1) Citation1-4 constitute interesting targets for the design of pharmacological agents useful in the treatment or prevention of a variety of disorders such as glaucoma, acid-base disequilibria, epilepsy and other neuromuscular diseases, altitude sickness, edema, and obesity [Citation5,Citation6]. A quite new and unexpected application of CA inhibitors (CAIs) regards their potential use in the management (imaging and treatment) of hypoxic tumors Citation7-14, since at least two CA isozymes of the 15 presently known in humans, i.e., CA IX and XII, are predominantly found in tumor cells and lack (or are present in very limited amounts) in normal tissues Citation15-18. The involvement of these enzymes, which catalyze the simplest physiological reaction, CO2 hydration to bicarbonate and a proton, in many physiological/pathological processes as well as the fact that, generally, different isozymes of the 15 mentioned above are involved in such particular processes, allows for the development of diverse medicinal chemistry applications for their inhibitors [Citation1,Citation2]. Thus, as mentioned above, the human isozymes hCA IX and hCA XII are the targets for the development of novel antitumor therapies Citation5Citation7-10, hCA II and XII for the development of antiglaucoma drugs Citation19-22, hCA Va and hCA Vb for the design of new anti-obesity agents [Citation6,Citation23,Citation24], hCA VII for the development of anticonvulsant/antiepileptic drugs [Citation25], whereas non-vertebrate CAs, such as for example the α-CA present in Plasmodium falciparum (pfCA) may lead to novel types of antimalaria drugs [Citation26] and the enzyme from the ulcer-producing bacteria Helicobacter pylori has been recently shown to be involved in the acclimitisation of the pathogen in the highly acidic medium within the stomach [Citation27], to cite only the most important isozymes investigated to-date for drug design purposes.
The α-CA gene family is present all over the phylogenetic tree, starting from bacteria and plants, protozoa, or invertebrates and ending with higher vertebrates including humans Citation5Citation28-31. The ubiquity of these enzymes in all these organisms is clearly due to their involvement in basic physiological processes, in which the three chemical species mentioned above (carbon dioxide, bicarbonate and the H+ ions) are involved. However, except for the human isozymes [Citation32] previously mentioned and some of the corresponding murine ones [Citation32,Citation33] as well as other such enzymes recently isolated and characterized in several bacterial/protozoa speciesCitation5Citation28-31, CAs from other organisms have only rarely been isolated, characterized and investigated for their interaction with sulfonamide inhibitors. Continuing investigations in the field of such exotic CAs (in a previous report [Citation34] the interaction of some sulfonamides with rainbow trout CA was investigated), we report here the purification and inhibition study with a group of 25 sulfonamides (some of which are used clinically) of the red blood cell CA from ostrich (Struthio camelus), a bird species with an increasing use in human alimentation, due to its favourable fatty acid profile and low intramuscular fat content [Citation35].
2 Materials and methods
2.1 Preparation of hemolysate
Two female ostriches (Struthio camelus) weighing 78 kg and 95 kg, respectively, aged 4 years, were used for the experiments. Blood samples from these birds were anticoagulated with ACD (Acid-citrate-dextrose) and the erythrocytes centrifuged at 1848 × g for 20 min at 4°C. The supernatant was removed, and the packed red cells were washed with NaCl (0.9%). The erythrocytes were then hemolysed with cold water. The ghost and intact cells were removed by centrifugation at 18,924 × g for 25 min at 4°C and the pH of the hemolysate was adjusted to 8.5 with solid Tris-base. The hemolysate was applied to an affinity column containing Sepharose-4B-l-tyrosine-sulfonamide [Citation36,Citation37] and equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.5). The affinity gel was washed with a solution of 25 mM Tris–HCl/22 mM Na2SO4 (pH 8.5). The ostrich enzyme (scCA) was eluted with a solution of 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6).
2.2 Determination of protein content
After scanning at 280 nm the tubes with significant absorbance were pooled and a quantitative protein determination was done by the Coomassie brilliant blue G-250 method [Citation35,Citation36].
2.3 Carbonic anhydrase assay
An SX.18MV-R Applied Photophysics stopped-flow instrument was used for measuring the initial velocities for the CO2-hydration reaction catalysed by different CA isozymes, including the newly purified scCA, by following the change in absorbance of a pH indicator [Citation38]. Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (pH 7.5) as buffer, 0.1 M Na2SO4 (for maintaining the ionic strength constant) and following the CA-catalyzed CO2-hydration reaction for a period of 10–100 s. Saturated CO2 solutions in water at 20°C were used as substrate. The CO2 concentrations ranged from 1.7 – 17 mM for the determination of the catalytic and inhibition constants. For each inhibitor at least six traces of the initial 5–10% of the reaction were used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitors were prepared at a concentration of 1–3 mM (in DMSO-water 1:1, v/v) and dilutions up to 0.01 nM done with the assay buffer mentioned above. The kinetic constants, kcat and kcat/KM, were obtained by non-least square methods using SigmaPlot, whereas the inhibition constants were obtained by non-linear least-squares methods using PRISM 3, from Lineweaver-Burk plots, as reported earlier [Citation26], and represent the mean from at least three different determinations.
2.4 Sulfonamide inhibitors
Acetazolamide (AZA), ethoxzolamide (EZA) and sulfanilamide (SA) were from Sigma-Aldrich (Milan, Italy). Compounds 1–5 used in the assay were previously reported by one of our groups Citation24Citation39-44.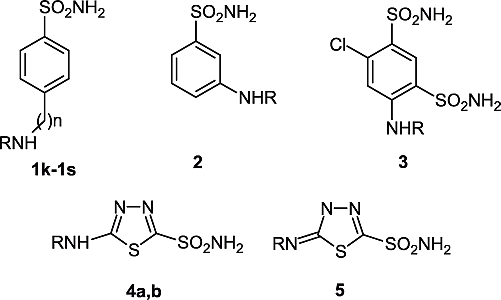
3 Results and discussion
Among the higher vertebrates CAs, the human and mouse isozymes have generally been thoroughly characterized both from the point of view of their kinetic properties for the CO2-hydration reaction, as well as for their interaction with sulfonamide inhibitorsCitation1-12. However, few isozymes of other origin have been investigated in detail. This is particularly true for birds (Aves) for which different reports mention the role of CAs in eggshell formation for chicken (Gallus gallus) Citation45-48 and ostrich (Struthio camelus) [Citation49], but no attempts to isolate, characterize and inhibit these enzymes have been done. Here we report the purification of the red blood cell CA isozyme from ostrich as well as the first inhibition study of this enzyme with a series of aromatic and heterocylic sulfonamides. This study is on the one hand of interest in order to understand potential differences between diverse CAs from different higher vertebrates such as mammals and birds, which diverged evolutionarily more than 350 million years ago. On the other hand, the increasing use of ostrich meat in human alimentation[Citation35,Citation50] and the fact that these birds are sometimes treated with various pharmacological agents (such as sulfonamides) raises the question as to whether the birds possess the same type of response to these agents as mammals, which are the species mostly used in the pharmacological evaluation of drugs. For example, a recent report showed that ostriches, in contrast to mammals, have a very fast elimination rate for several non-steroidal anti-inflammatory drugs[Citation51].
We isolated Struthio camelus red blood cells CA (abbreviated as scCA) from ostrich blood by means of an original procedure (see Materials and methods for details). In contrast to humans and more generally primates, Struthio camelus has only one blood CA isozyme [Citation1]. The ostrich enzyme has been purified to homogeneity as observed from PAGE (data not shown) and its kinetic parameters were measured using the CO2-hydration reaction and a stopped-flow technique [Citation32,Citation38]. As seen from data of , similarly to the human red blood cell isozyme II, scCA shows a high activity for the physiological reaction with kcat of 1.2·106 s− 1 and kcat/KM of 1.8. 107 M− 1·s− 1. Thus, this enzyme (scCA) is more active than the slow red blood cell human isozyme hCA I, and much more active than the mouse isozyme mCA XIII (the last cytosolic CA recently characterized in detail) [Citation32] or the human isozyme hCA III (which is one of the least active CAs) [Citation1]. On the other hand, data in also show a rather different affinity of these isozymes for acetazolamide, the sulfonamide CA inhibitor par excellence. The inhibition data will be presented in detail later ().
Table I. Kinetic parameters for CO2-hydration reaction catalysed by the cytosolic α-CA isozymes of mammalian and avian origin and their susceptibility to acetazolamide inhibition.
Table II. hCA I, II and scCA inhibition data with sulfonamides 1–5, acetazolamide (AZA), ethoxzolamide (EZA) and sulfanilamide (SA). Data for hCA I and II are from refs. Citation24Citation39-44.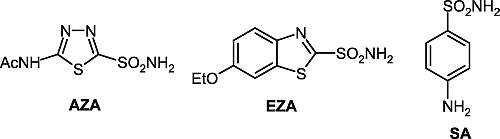
The interaction was investigated of scCA with three sulfonamide drugs known to possess relevant CA inhibitory properties [Citation1], acetazolamide AZA, ethoxzolamide EZA and sulfanilamide SA, as well as with a library of sulfonamides of types 1–5, reported earlier by one of our groups Citation24Citation39-44. Most of these sulfonamides are aromatic derivatives of sulfanilamide, homosulfanilamide or 4-aminoethylbenzenesulfonamide (derivatives 1p–1s), to which various acyl-, alkyl/arylsulfonyl-, or (thio)ureido- tails have been attached at the primary amino group. Several other derivatives were included in our study, such as for example the metanilamide 2, the benzene-1,3-disulfonamide derivative 3, as well as the thiadiazole/thiadiazoline-2-sulfonamides 4 and 5, in order to obtain a detailed inhibition profile of this new CA. The inhibition data for the human red blood cell isozymes hCA I and II are also included in for comparative reasons, although these data were reported earlier Citation24Citation39-44.
The following should be noted regarding inhibition of scCA with this series of sulfonamides: (i) a group of sulfonamides among the derivatives investigated here, including 1a–1c, 1h, 1j, 1k, 1n, 1p, 1r and 2 showed weak scCA inhibitory properties, with KI values in the range 2430–4700 nM. These sulfonamides were generally much more potent hCA II inhibitors (KI = 4–246 nM), and typically weaker hCA I inhibitors (KI = 3300–21400 nM for derivatives 1a to 1h). It may be seen however, that some of these sulfonamides showed better hCA I than scCA inhibitory properties; (ii) several other derivatives, such as 1d–1 g, 1m, 5, AZA and SA, showed moderate scCA inhibitory properties, with KI values in the range 260–768 nM. Again, these sulfonamides showed a completely different inhibition profile against the human isozymes hCA I and II. Thus, the first derivatives mentioned above (1d–1g, 1m) act as moderate hCA II inhibitors and rather weak hCA I inhibitors (except 1m which is a moderate hCA I inhibitor). Acetazolamide and the furan-substituted sulfonamide 5 were, on the other hand, very potent hCA II and weaker hCA I inhibitors, whereas they did not show impressive scCA inhibitory properties. Sulfanilamide is a very weak hCA I inhibitor and a moderate-weak inhibitor of hCA II and scCA; (iii) the best scCA inhibitors among the investigated sulfonamides were derivatives 1i, 1q, 1s, 3, 4 and EZA, which showed KI values in the range 3.9–72 nM. Obviously, ethoxzolamide is a particularly potent inhibitor of the ostrich enzyme, for which it has a higher affinity than for the two human isozymes included in our study. Most of these compounds were however, much more potent inhibitors of the human cytosolic isozymes hCA I and II (some of them, such as 1s, 4a and 4b in the low nanomolar range). Considering the rather heterolgeneous series of compounds investigated, it is apparent that potent scCA inhibitors may be obtained both from the aromatic series (such as derivatives 1i, 1q, 1s and 3) as well as from the heterocyclic sulfonamides (4a,b and EZA).
In conclusion, we report the purification of the red blood cell CA from ostrich (scCA), its kinetic parameters for the CO2-hydration reaction, as well as an inhibition study of this enzyme with a series of aromatic and heterocylic sulfonamides. The ostrich enzyme showed an inhibition profile quite different from that of the human red blood cell cytosolic isozymes hCA I and II. scCA has generally a lower affinity for sulfonamide inhibitors as compared to hCA I and II. The only sulfonamide which behaved as a very potent inhibitor of this enzyme was ethoxzolamide (KI = 3.9 nM) whereas acetazolamide and sulfanilamide behaved as weaker inhibitors (KI = 303–570 nM). Several other aromatic and heterocyclic sulfonamides showed good affinities for the ostrich enzyme, with KI values in the range 25–72 nM.
Acknowledgements
During the course of this study, the Scientific and Technical Research Council of Turkey (TUBITAK) provided a scholarship (NATO-A2) to Ozen Ozensoy, which is gratefully acknowledged. The authors also thank Balikesir University, Research Center of Applied Sciences (BURCAS/Balikesir, Turkey) for providing the research facilities.
References
- Supuran CT, Scozzafava A, Conway J. Carbonic anhydrase—Its Inhibitors and Activators. CRC Press, Boca Raton, London, New York 2004; 1–363
- Scozzafava A, Mastrolorenzo A, Supuran CT. Expert Opin Ther Pat 2004; 14: 667–702
- Supuran CT. Carbonic anhydrase—Its inhibitors and activators, CT Supuran, A Scozzafava, J Conway. CRC Press, Boca Raton 2004; 1–23
- Supuran CT, Vullo D, Manole G, Casini A, Scozzafava A. Curr Med Chem Cardiovasc Hematol Agents 2004; 2: 49–68
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. J Enz Inhib Med Chem 2004; 19: 199–229
- Supuran CT. Expert Opin Ther Pat 2003; 13: 1545–1550
- Svastova E, Hulikova A, Rafajova M, Zat'ovicova M, Gibadulinova A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, Pastorekova S. FEBS Lett 2004; 577: 439–445
- Supuran CT. Expert Opin Investig Drugs 2003; 12: 283–287
- Supuran CT, Scozzafava A, Casini A. Med Res Rev 2003; 23: 146–189
- Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Curr Med Chem 2003; 10: 925–953
- Vullo D, Franchi M, Gallori E, Pastorek J, Scozzafava A, Pastorekova S, Supuran CT. J Enz Inhib Med Chem 2003; 18: 403–406
- Vullo D, Franchi M, Gallori E, Pastorek J, Scozzafava A, Pastorekova S, Supuran CT. Bioorg Med Chem Lett 2003; 13: 1005–1009
- Winum JY, Vullo D, Casini A, Montero JL, Scozzafava A, Supuran CT. J Med Chem 2003; 46: 5471–5477
- Winum JY, Vullo D, Casini A, Montero JL, Scozzafava A, Supuran T. J Med Chem 2003; 46: 2197–2204
- Potter C, Harris L. Cell Cycle 2004; 3: 164–167
- Pastorekova S, Casini A, Scozzafava A, Vullo D, Pastorek J, Supuran T. Bioorg Med Chem Lett 2004; 14: 869–873
- Rafajova M, Zatovicova M, Kettmann R, Pastorek J, Pastorekova S. Int J Oncol 2004; 24: 995–1004
- Robertson N, Potter C, Harris AL. Cancer Res 2004; 64: 6160–6165
- Scozzafava A, Briganti F, Mincione G, Menabuoni L, Mincione F, Supuran CT. J Med Chem 1999; 42: 3690–3700
- Supuran CT, Scozzafava A, Menabuoni L, Mincione F, Briganti F, Mincione G. Eur J Pharm Sci 1999; 8: 317–328
- Scozzafava A, Menabuoni L, Mincione F, Briganti F, Mincione G, Supuran CT. J Med Chem 1999; 42: 2641–2650
- Vullo D, Innocenti A, Nishimori I, Pastorek J, Scozzafava A, Pastorekova S, Supuran CT. Bioorg Med Chem Lett 2005; 15: 963–969
- Innocenti A, Antel J, Wurl M, Scozzafava A, Supuran CT. Bioorg Med Chem Lett 2004; 14: 5703–5707
- Vullo D, Franchi M, Gallori E, Antel J, Scozzafava A, Supuran CT. J Med Chem 2004; 47: 1272–1279
- Vullo D, Voipio J, Innocenti A, Rivera C, Ranki H, Scozzafava A, Kaila K, Supuran T. Bioorg Med Chem Lett 2005; 15: 971–976
- Krungkrai J, Scozzafava A, Reungprapavut S, Krungkrai SR, Rattanajak R, Kamchonwongpaisan S, Supuran CT. Bioorg Med Chem 2005; 13: 483–489
- Marcus EA, Moshfegh AP, Sachs G, Scott R. J Bacteriol 2005; 187: 729–738
- Hewett-Emmett D, Tashian E. Mol Phylogenet Evol 1996; 5: 50–77
- Reungprapavut S, Krungkrai SR, Krungkrai J. J Enz Inhib Med Chem 2004; 19: 249–256
- Elleby B, Chirica LC, Tu C, Zeppezauer M, Lindskog S. Eur J Biochem 2001; 268: 1613–1619
- Chirica LC, Petersson C, Hurtig M, Jonsson BH, Boren T, Lindskog S. Biochim Biophys Acta 2002; 1601: 192–199
- Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT, Parkkila AK, Saarnio J, Kivela AJ, Waheed A, Sly WS, Parkkila S. J Biol Chem 2004; 279: 2719–2727
- Whittington DA, Grubb JH, Waheed A, Shah GN, Sly WS, Christianson W. J Biol Chem 2004; 279: 7223–7228
- Bulbul M, Hisar O, Beydemir S, Ciftci M, Kufrevioglu I. J Enz Inhib Med Chem 2003; 18: 371–375
- Viljoen M, Hoffman LC, Brand TS. Meat Sci 2005; 69: 255–261
- Cakir U, Ugras HI, Ozensoy O, Sinan S, Arslan O. J Enz Inhib Med Chem 2004; 19: 257–261
- Ozensoy O, Arslan O, Sinan SO. Biochemistry (Mosc) 2004; 69: 216–219
- Khalifah RG. J Biol Chem 1971; 246: 2561–2573
- Clare BW, Supuran CT. Eur J Med Chem 1999; 34: 463–474
- Supuran CT, Scozzafava A, Jurca BC, Ilies MA. Eur J Med Chem 1998; 33: 83–93
- Casini A, Scozzafava A, Mincione F, Menabuoni L, Supuran CT. J Enz Inhib Med Chem 2002; 17: 333–343
- Supuran CT, Ilies MA, Scozzafava A. Eur J Med Chem 1998; 33: 739–752
- Ilies M, Supuran CT, Scozzafava A, Casini A, Mincione F, Menabuoni L, Caproiu MT, Maganu M, Banciu D. Bioorg Med Chem 2000; 8: 2145–2155
- Mincione F, Starnotti M, Menabuoni L, Scozzafava A, Casini A, Supuran CT. Bioorg Med Chem Lett 2001; 11: 1787–1791
- Kadoya Y, Nagahama S, Kuwahara H, Shimazaki M, Shimazu A, Ogawa Y, Yagi T. Osaka City Med J 1987; 33: 111–119
- Palatroni P, Gabrielli MG, Grappasonni I. Anat Anz 1987; 163(1)5–18
- Quelo I, Machuca I, Jurdic P. J Biol Chem 1998; 273: 10638–10646
- Gabrielli MG, Cox JV, Materazzi G, Menghi G. Histochem Cell Biol 2004; 121: 189–199
- Holm L, Ruziwa SD, Dantzer V, Ridderstrale Y. Br Poult Sci 2000; 41: 244–249
- Musara C, Chamunorwa J, Holtug K, Skadhauge E. Br Poult Sci 2003; 44: 316–326
- Baert K, De Backer P. Comp Biochem Physiol C 2003; 134: 25–33