Abstract
Effects of nicotine, and nicotine + vitamin E on glucose 6-phosphate dehydrogenase (G-6PD) activity in rat muscle, heart, lungs, testicle, kidney, stomach, brain and liver were investigated in vivo and in vitro on partially purified homogenates. Supplementation period was 3 weeks (n = 8 rats per group): nicotine [0.5 mg/kg/day, intraperitoneal (ip)]; nicotine + vitamin E [75 mg/kg/day, intragastric (ig)]; and control group (receiving only vehicle). The results showed that nicotine (0.5 mg/kg, ip) inhibited G-6PD activity in the lungs, testicle, kidney, stomach and brain by 12.5% (p < 0.001), 48% (p < 0.001), 20.8% (p < 0.001), 13% (p < 0.001) and 23.35% (p < 0.001) respectively, and nicotine had no effects on the muscle, heart and liver G6PD activity. Also, nicotine + vitamin E inhibited G-6PD activity in the testicle, brain, and liver by 32.5% (p < 0.001), 21.5% (p < 0.001), and 16.5% (p < 0.001) respectively, and nicotine + vitamin E activated the muscle, and stomach G-6PD activity by 36% (p < 0.05), and 20% (p < 0.001) respectively. In addition, nicotine + vitamin E did not have any effects on the heart, lungs, and kidney G-6PD activity. In addition, in vitro studies were also carried out to elucidate the effects of nicotine and vitamin E on G-6PD activity, which correlated well with in vivo experimental results in lungs, testicles, kidney, stomach, brain and liver tissues. These results show that vitamin E administration generally restores the inactivation of G-6PD activity due to nicotine administration in various rat tissues in vivo, and also in vitro.
Introduction
Glucose 6-phosphate dehydrogenase (G-6PD) catalyzes the first step of the pentose phosphate metabolic pathway, which is the unique source of NADPH synthesis in cells Citation1-4. The most important role of NADPH in cells is regeneration of reduced glutathione, which prevents protein denaturation, preserves the integrity of the cell membrane sulfhydryl groups, and detoxifies peroxides and oxygen free radicals in the cells [Citation1,Citation5]. But NADPH production is decreased in G-6PD deficiency.
G-6PD deficiency is an X-linked hereditary disorder and approximately 400 million persons are affected from this; it is fully expressed in males and homozygote females. Some drugs and chemicals (primaquine, aspirin, sulfonamids etc.) used lead to production and accumulation of toxic peroxides, and cause oxidation of proteins and lipids in cell membranes [Citation1].
G-6PD deficiency is frequently seen in African, Mediterranean, Middle East and Far East nations and their lineages with a frequency ranging from 5% to 40% Citation5-7. In Turkey, cases of this disorder appeared in the Çukurova Region and Başkale district of Van and the highest incidence is seen in the Jewish Kurd population (62% of males) [Citation8].
The formation of the reactive oxygen species (ROS) in cells leads to the formation of free radicals in metabolic processes. These harmful species cause damages to many molecules such as lipids, proteins and nucleic acids. These harmful effects are controlled by the antioxidant defence system in cells. The most important free radical chain-breaking molecule in the antioxidant defence system in various tissues of the body is glutathione Citation9-13. Furthermore, the enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GR) and G-6PD are necessary to remove these radicals and keep the cells stable. Under normal conditions, the reductive and oxidative capacity of the cell (redox state) is in favor of the oxidation Citation14-16. However, ROS produced in oxidative stress are removed by the antioxidant defence system. A number of drugs and chemicals increase the ROS production in specific organs of the body. Many researchers have determined that nicotine contributes to ROS production Citation17-20. Cigarette smoking is common in many societies. Two thirds of American adults are addicted to alcohol and 30% of them are addicted to both cigarettes and alcohol Citation21-24. Nicotine, the major toxic component of cigarette smoke Citation25-29, is a risk factor for various cardiovascular diseases and cancer. Kessler et al. [Citation30] have determined a marked increase in nicotine content in all kinds of cigarettes in the last decade in the United States. Shaw et al. [Citation31] reported that one cigarette decreases lifespan by 11 minutes. Nicotine is oxidized to its metabolite cotinine, which has a long half life and may contribute to vascular diseases Citation31-33. Half lives of nicotine and its metabolite cotinine are 1.3–2.7 and 15–19 hours respectively [Citation34,Citation35].
Decreases in the cytochrome P450 IIE1 due to chronic nicotine treatment reported increases free radical formation and leads to oxidative damage in rats Citation19Citation24Citation29Citation36-38.
Here we used vitamin E to ascertain whether it counteracted nicotine-induced adverse effects on G-6PD activity. Vitamin E is well accepted as nature's most effective lipid-soluble, chain-breaking antioxidant, protecting cell membranes from peroxidative damage [Citation39].
Owing to the widespread use of cigarettes, we thought it was important to examine the effect of nicotine on G-6PD activity, and for this purpose, we investigated the in vivo effects of nicotine and nicotine + vitamin E on G-6PD activities in the rat muscle, heart, lungs, testicle and liver in vivo, and we also carried out in vitro enzyme inhibition experiments. Effects of many chemicals and drugs on red cell G-6PD enzyme activity have been investigated, such as ethanol, halothane, isoflurane, ketamine, sevoflurane, prilocaine, diazepam, midazolam, dantrolene sodium, theophylline, lidocaine, cyclophosphamide, hyoscine N-butyl bromide, tranexamic acid, cytarabine, sodium ceftizoxime, sodium ampicillin, sodium cefuroxime, sodium cefazolin, sodium cefoperazone, streptomycin sulphate, gentamicin sulphate, netilmicin sulphate, melatonin, dexamethasone, metamizol, magnesium sulfate, putrescine, spermidine, kinetin, abscisic acid, vancomycin, cefazolin, and ceftriaxone Citation40-49, but no studies on other tissues have been encountered in previous reports.
Materials and methods
Materials
NADP+, glucose 6-phosphate, protein assay reagent were purchased from Sigma Chem. Co. Germany. All other chemicals used were of analytical grade and were purchased from either Sigma or Merck.
Animals
Twenty-four rats (Sprague-Dawley strain, weight = 225 ± 28 g), fed with standard laboratory chow and water, were used in the study. They were randomly divided into 3 groups (8 rats per group) and placed in separate cages during the study. The groups were as follows:
Group I: Nicotine (0.5 mg/kg/day, ip); Group II: Nicotine (0.5 mg/kg/day, ip) + Vitamin E (75 mg/kg/day, ig); Group III: Control group (received only the same amounts of vehicles, 0.9% NaCl solution, ip, and corn oil, ig). Supplementation period was 3 weeks. Animal experimentations were carried out in an ethically manner by following guidelines as set by the Ethical Committee of Ataturk University.
Preparation and administration of nicotine
Hydrogen tartrate salt of nicotine (Sigma N-5260) was dissolved in 0.9% NaCl solution to give a 0.15 mg/ml concentration of nicotine. Then the nicotine solution was adjusted to pH 7.4 by 0.1 N NaOH. Nicotine (0.5 mg/kg/day) was administered by ip injection to groups I and II for 3 weeks.
Preparation and administration of vitamin E
Vitamin E (Ephynal 300 mg capsule, Roche, France) was dissolved in corn oil (30 mg/ml) and administered orally by a stomach tube (approximately 75 mg/kg/day) to group II for 3 weeks.
Sample collection
At the end of the experiment, the animals were anaesthetized with ketamine-HCl (Ketalar, Eczacibasi, Turkey, 20 mg/ kg, ip). The animals were killed by exsanguination by cardiac puncture after thoracotomy. Then, each tissue was carefully removed, rinsed in saline and stored at − 80oC until homogenization.
Preparation of homogenate
A piece of each tissue (approximately 300 mg) was homogenized by an OMNI TH International, model TH 220 (Warrenton, VA 20187 USA) homogenizer in 20 mM Tris-HCl, pH 7.4 (1/10 W/V) on ice for 10 s at the first speed level. Then, the homogenates were centrifuged at 10 000 × g for 15 min at 4°C. The supernatants were stored at − 80°C in aliquots until biochemical measurements.
Ammonium sulphate fractionation and dialysis
Ammonium sulphate (20–60%) precipitation was made on the homogenate. Ammonium sulphate was slowly added for complete dissolution. The suspension was centrifuged at 5000 × g for 15 min and precipitate was dissolved in 20 mM Tris-HCl (pH 7.4), then dialysed at 4°C in 20 mM Tris-HCl (pH 7.4) for 2 h with two changes of buffer. Thus, partially purified total G-6PD was obtained by ammonium sulphate fractionation and dialysis from tissue homogenates (muscle, heart, lungs, testicle, kidney, stomach, brain and liver tissues).
Activity and protein determination
G-6PD was measured spectrophotometrically at 25°C as described by Beutler [Citation50]. Briefly, the enzyme sample was added to a 2.5 ml of final volume incubation mixture containing 0.1 M Tris-HCl+0.5 mM EDTA pH 8.0, 10 mM MgCl, 0.2 mM NADP+ and 0.6 mM glucose-6-phosphate. The activity measurement was done by monitoring the increase in absorption at 340 nm due to the reduction of NADP+ at 25°C. One EU was defined as the enzyme reducing 1μmol NADP+ per min at 25°C and optimal pH (pH 8.0).
Protein was determined by Bradford's method [Citation51] by using bovine serum albumin as standard. The enzymatic activity and protein content were measured in 1 ml of each sample of enzyme. Then, enzyme activity was determined as EU/mg of protein.
In Vitro Studies
In vitro effects of nicotine (5 mM), nicotine (5mM)+vitamin E (10 mM) on partially purified total G-6PD from tissues homogenates were investigated. Activies were measured by adding 20, 40, 60, 80, and 100 μL of 5 mM nicotine, and nicotine (5mM)+vitamin E (10 mM). Control cuvette activity was accepted as 100%. Each tissue homogenate from each rat was studied separately in triplicate. CV%s were within 5%.
Statistical analysis
One-way ANOVA with post-hoc LSD test was used to compare the group means and p < 0.05 was considered statistically significant. SPSS-for Windows (version 10.0) was used for statistical analyses.
Results
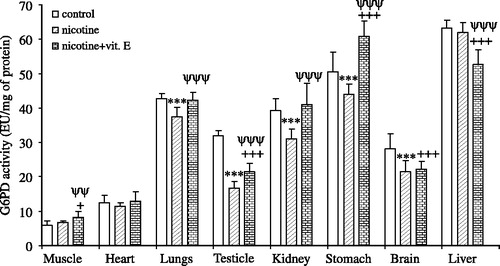
Nicotine inhibited G-6PD activity in the lungs, testicle, kidney, stomach and brain by ∼12.5% (p < 0.001), ∼48% (p < 0.001), ∼20.8% (p < 0.001), ∼13% (p < 0.001), and ∼23.35% (p < 0.05) respectively, however, it had no effect on the muscle, heart and liver G-6PD activity (). On the other hand, nicotine+vitamin E activated the muscle, and stomach G-6PD enzyme activity by ∼36% (p < 0.05), and ∼20% (p < 0.001), respectively (). However, nicotine + vitamin E inhibited the testicle, brain and liver G-6PD enzyme activity by ∼32.5% (p < 0.001), ∼21.5% (p < 0.001) and ∼16.5% (p < 0.001) respectively, and had no effects on heart, lungs and kidney G-6PD activity in vivo ().
Figure 1 In vivo effects of nicotine and nicotine + vitamin E on glucose 6-phosphate dehydrogenase activity in rat muscle, heart, lungs, testicle, kidney, stomach, brain, and liver tissue. Signicifant, *nicotine vs. control, +nicotine + vitamin E vs. control, ψnicotine + vitamin E vs. nicotine. Significant *p, +p, and ψp < .05; **p, ++p, and ψψp < .02; ***p, +++p, and ψψψp < .001, n = 8.
The results of the in vitro inhibition studies with nicotine (5 mM) and nicotine (5mM)+vitamin E (10 mM) are shown in .
Table I. In vitro effects of nicotine and nicotine + vitamin E on glucose 6-phosphate dehydrogenase activity in the various rat tissues.
Discussion
Many drugs and chemicals have adverse or beneficial effects on human enzymes and metabolic processes. For example, a diuretic drug, acetazolamide, inhibits carbonic anhydrase [Citation5]. Inhibition of some important enzymes, which play key roles in metabolic pathways, may result in pathologic conditions or disorders. G-6PD plays an important role in metabolism and has a vital function in various tissues. Metabolic diseases such as diabetes mellitus affect some enzyme activities [Citation52] and it has been shown that some chemicals and drugs also inhibit G-6PD activity. For example, incubation of the major product of lipid peroxidation 4-hydroxy 2-nonenal with G-6PD inhibits enzyme activity rapidly [Citation53]. In addition, epiandrosterone inhibits erythrocyte G-6PD uncompetitively and suppresses hexose monophosphate shunt activity by more than 95% [Citation54].
Glutathione levels in liver and testicles decrease markedly after chronic nicotine treatment. Nicotine is oxidized to its main metabolite cotinine in liver and causes the formation of free radicals in tissues. The formation of these radicals causes oxidative damage. The decrease in GSH in tissues leads to oxidative tissue damage [Citation55].
There are many studies about the effects of nicotine on enzyme activities. For example, inhibition of kidney and testes SOD and activation of liver SOD in nicotine- treated rats has been shown. Inhibition of liver CAT and activation of kidney, lung and testes CAT in nicotine-treated rats has been reported [Citation23].
Gumustekin et al. [Citation56] have reported that nicotine increased the activity of GSH-Px of the brain while vitamin-E abolished this effect. Nicotine also inhibited brain GST activity and this activity was restored by vitamin E, too. In addition, Suleyman et al. [Citation57] have reported that nicotine inhibited the activities of GSH-Px and SOD of erythrocytes while vitamin-E reversed these effects.
The increased mRNA levels of endothelial nitric oxide synthase by nicotine [Citation58], inhibition of tryptophan hydroxylase in alcohol and nicotine-treated rats [Citation59], while activation of tryptophan hydroxylase by nicotine (1 mg/kg) were reported [Citation60].
Activation of the liver metabolism enzymes in rats, which were exposed to cigarette smoke [Citation61], and the increased (2.5 fold) expression of enzymes involved in energy metabolism in nicotine-treated rats [Citation62] have been found. In addition, activation of adenylate cyclase in nicotine (6 mg/kg)-treated rats has been demonstrated [Citation63].
In this study, nicotine inhibited G-6PD activity in the lungs, testicle, kidney, stomach and brain tissues in vivo was compared with the control group. Nicotine can cause competitive inhibition by binding at the active site of G-6PD. It also possible that it can cause non-competitive inhibition by binding to other sites affecting the three dimentional structure of the enzyme [Citation4]. However, it had no effect on muscle, heart and liver G-6PD activity. Administration of vitamin E with nicotine prevented this inhibition in the lungs, testicle, kidney, stomach and brain. In the stomach, this activity was even higher than the control group. However, nicotine + vitamin E had no effects on the heart, lungs, and kidney G-6PD activity compared with the control group.
As seen in , nicotine moderately inhibited G-6PD activity in vitro, in lung, testicle, kidney, stomach and brain tissues and this inhibition was eliminated by vitamin E. In vitro G-6PD inhibition results correlate well with in vivo experimental results in muscle, heart, lung, testicle, kidney, stomach and brain tissues. In in vivo study, when the average volume of blood present in a rat is accepted to be about 15 ml, 0.5 mg/kg dose corresponds to 0.0513 mM nicotine concentration. In in vitro study, enzyme activities were determined by using 0.04, 0.08, 0.12, 0.16 and 0.20 mM cuvette concentrations. Since similar nicotine concentrations have been used in both in vivo and in vitro experiments, our results obtained by in vitro and in vivo studies were comparable. However, in vitro and in vivo results did not correlate in liver tissue. Although it is difficult to envisage how nicotine, vitamine E modifies the G-6PD activity in various tissues in vitro, the inhibition of G-6PD by nicotine may result from binding to the active site of G6PD in heart, lung, stomach, brain and liver. On the other hand, facilitating proton transfer may be responsible for the increased G-6PD activity, which may be a different isoenzyme of G-6PD, in liver tissue [Citation64].
The results of this study show that vitamin E administration may generally restore G-6PD activity inactivated due to nicotine administration in various tissues in vivo, and also in vitro.
References
- Beutler E. Glucose 6-phosphate dehydrogenase deficiency and other enzyme abnormalities. Williams Hematology, E Beutler, MA Lichtman, BS Coller, TJ Kipps. Mc Graw-Hill Co., New York 1995; 564–581
- Deutsch J. Glucose-6-phosphate dehydrogenase. Methods of enzymatic analysis, HU Bergmeyer, J Bergmeyer. VCH Verlagsgerellschaff, Weinheim 1983; 190–196
- Keha EE, Kufrevioglu OI. Biochemistry (in Turkish). Safak Yayinevi, Erzurum 1997
- Lehninger AL, Nelson DL, Cox MM. Principles of biochemistry. Worth Publishers, New York 1993
- Warnock DG. Diuretic agents. Basic and clinical pharmacology, BG Katzung. Appletion and Lange, USA 1989; 183–197
- Berkow R. The Merck manual of diagnosis and therapy. Merck & Co. Inc, Rahway, N.J., USA 1987
- Laurence DR, Bennett PN, Brown MJ. Clinical Pharmacology. Churchill Livingstone, Singapore 1997
- Kayaalp SO. Medical pharmacology, in terms of rational treatment (Rasyonel tedavi yonunden tibbi farmakoloji). Hacettepe-Tas Ltd.Sti, Ankara 1998
- Ames BN, Shigenaga MK, Hagen TM. Proc Natl Acad Sci USA 1993; 90: 7915–7922
- Bondy SC, Orozco J. Alcohol Alcohol 1994; 29: 375–383
- DeLeve LD, Kaplowitz N. Pharmacol Ther 1991; 52: 287–305
- Gul M, Kutay FZ, Temocin S, Hanninen O. Indian J Exp Biol 2000; 38: 625–634
- McCord JM. Clin Biochem 1993; 26: 351–357
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Clarendon Press, Oxford 1989
- Rubin E. Alcohol Health Res World 1993; 17: 272–278
- Somani SM. Exercise, drugs and tissue specific antioxidant system. Pharmacology in exercise and sports, SM Somani. CRC Press, Boca Raton FL 1996; 57–95
- Guemouri L, Lecomte E, Herbeth B, Pirollet P, Paille F, Siest G, Artur Y. J Stud Alcohol 1993; 54: 626–629
- Jenkins RR, Goldfarb A. Med Sci Sports Exer 1993; 25: 210–212
- Leanderson P, Tagesson C. Chem Biol Interact 1992; 81: 197–208
- Wetscher GJ, Bagchi M, Bagchi D, Perdikis G, Hinder PR, Glaser K, Hinder RA. Free Radic Biol Med 1995; 18: 877–882
- Mehta MC, Jain AC, Billie M. J Cardiovasc Pharmacol 1998; 31: 930–936
- US Department of Health and Human Services. Seventh Special Report to the US Congress on Alcohol and Health. National Institute on Alcohol Abuse and Alcoholism, Washington, DC 1990, (US Department of Health and Human Services Publication ADM 90, 1656)
- US Environmental Protection Agency. Respiratory Health Effects of Passive Smoking, Lung Cancer and Other Disorders. US Environmental Protection Agency, Washington, DC 1992, (US Environmental Protection Agency Publication 600, 6-90/006F)
- Weksler BB, Moore A, Tepler J. Hematology. Cecil essentials of medicine, TE Andreoli, CCJ Carpenter, F Plum, LHJ Smith. WB Saunders Co., Philadelphia 1990; 341–363
- Del Boccio G, Lapenna D, Porreca E, Pennelli A, Savini F, Feliciani P, Ricci G, Cuccurullo F. Atherosclerosis 1990; 81: 127–135
- Hoffmann D, Rivenson A, Hecht SS. Crit Rev Toxicol 1996; 26: 199–211
- Maser E. Trends Pharmacol Sci 1997; 18: 270–275
- McGinnis JM, Foege WH. JAMA 1993; 270: 2207–2212
- Pryor WA, Stone K. Ann NY Acad Sci 1993; 686: 12–27, discussion 27–18
- Kessler DA, Witt AM, Barnett PS, Zeller MR, Natanblut SL, Wilkenfeld JP, Lorraine CC, Thompson LJ, Schultz WB. N Engl J Med 1996; 335: 988–994
- Shaw M, Mitchell R, Dorling D. BMJ 2000; 320: 53
- Sastry BV, Chance MB, Singh G, Horn JL, Janson VE. Pharmacology 1995; 50: 128–136
- Sastry BV, Gujrati VR. Ann NY Acad Sci 1994; 714: 312–314
- Buccafusco JJ, Terry AV, Jr. Life Sci 2003; 72: 2931–2942
- Gilman AG, Goodman LS, Rall TW, Murad F. Goodman and Gilman's The pharmacological basis of therapeutics. MacMillan Publishing Co. 1980
- Anandatheerthavarada HK, Williams JF, Wecker L. J Neurochem 1993; 60: 1941–1944
- Ashakumary L, Vijayammal PL. J Ecotoxicol Environ Monit 1991; 1: 283–290
- Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. Biochem Pharmacol 1998; 56: 831–839
- Brigelius-Flohe R, Traber MG. Faseb J 1999; 13: 1145–1155
- Altikat S, Ciftci M, Buyukokuroglu ME. Pol J Pharmacol 2002; 54: 67–71
- Beydemir S, Gulcin I, Kufrevioglu OI, Ciftci M. Pol J Pharmacol 2003; 55: 787–792
- Beydemir S, Kulacoglu DN, Ciftci M, Kufrevioglu OI. Eur J Ophthalmol 2003; 13: 155–161
- Beydemir S, Kulacoglu DN, Ciftci M, Kufrevioglu OI. Turk J Chem 2003; 27: 601–608
- Buyukokuroglu ME, Altikat S, Ciftci M. Alcohol Alcohol 2002; 37: 327–329
- Ciftci M, Senyayla F, Ozdemir H, Buyukokuroglu ME. Pol J Pharmacol 2002; 54: 673–679
- Ciftci M, Bilici D, Kufrevioglu OI. Pharmacol Res 2001; 44: 7–11
- Ciftci M, Kufrevioglu OI, Gundogdu M, Ozmen I. Pharmacol Res 2000; 41: 109–113
- Ciftci M, Demir Y, Ozmen I, Atici O. J Enzyme Inhib Med Chem 2003; 18: 71–76
- Odcikin E, Ozdemir H, Ciftci M, Capoglu I. Endocr Res 2002; 28: 61–68
- Beutler E. Red cell metabolism: A manual of biochemical method. Grune and Stration, New York 1984
- Bradford MM. Anal Biochem 1976; 72: 248–254
- Gupta BL, Nehal M, Baquer NZ. Indian J Exp Biol 1997; 35: 792–795
- Friguet B, Stadtman ER, Szweda LI. J Biol Chem 1994; 269: 21639–21643
- Grossman S, Budinsky R, Jollow D. J Pharmacol Exp Ther 1995; 273: 870–877
- Husain K, Scott BR, Reddy SK, Somani SM. Alcohol 2001; 25: 89–97
- Gumustekin K, Altinkaynak K, Timur H, Taysi S, Oztasan N, Polat MF, Akcay F, Suleyman H, Dane S, Gul M. Hum Exp Toxicol 2003; 22: 425–431
- Suleyman H, Gumustekin K, Taysi S, Keles S, Oztasan N, Aktas O, Altinkaynak K, Timur H, Akcay F, Akar S, Dane S, Gul M. Biol Pharm Bull 2002; 25: 1133–1136
- Zhang S, Day I, Ye S. Atherosclerosis 2001; 154: 277–283
- Jang MH, Shin MC, Lee TH, Kim YP, Jung SB, Shin DH, Kim H, Kim SS, Kim EH, Kim CJ. Neurosci Lett 2002; 329: 141–144
- Lee TH, Jang MH, Shin MC, Lim BV, Choi HH, Kim H, Kim EH, Kim CJ. Nutr Res 2002; 22: 1445–1452
- Vanscheeuwijck PM, Teredesai A, Terpstra PM, Verbeeck J, Kuhl P, Gerstenberg B, Gebel S, Carmines EL. Food Chem Toxicol 2002; 40: 113–131
- Hu D, Cao K, Peterson-Wakeman R, Wang R. Biochem Biophys Res Commun 2002; 297: 729–736
- Abreu-Villaca Y, Seidler FJ, Slotkin TA. Brain Res 2003; 988: 164–172
- Supuran CT, Scozzafava A, Casini A. Med Res Rev 2003; 23: 146–189