Abstract
Oxovanadium(IV) -derived antifungals have been prepared by condensing equimolar amounts of vanadyl sulfate with hydrazides. All the synthesized ligands and their metal complexes were characterized by IR, UV–Visible and micro analytical data. These synthesized compounds were screened for their antifungal activity against Aspergillus flavus (A. flavus), Trichophyton longifusus (T. longifusus), Candida albicans (C. albicans), Microsporum canis (M. canis), Fusarium solani (F. solani) and Candida glaberata (C. glaberata) fungal strains. All complexes showed promising antifungal activity against different fungal strains with the exception of F. Solani and C. glaberata. Minimum Inhibitory Concentration (MIC) of different complexes and ligands are in the range of 250 to 400 μg/mL. Complex 7a and ligand 13 exhibit lowest MIC of 250 μg/mL whereas, complex 5a and ligands 2, 7 and 14 showed highest MIC of 400 μg/mL.
Introduction
Several studies have demonstrated that some antitumour drugs exhibited enhanced activity when administered in the form of their metal complexes [Citation1,Citation2]. In view of wide spread resistant strains of microorganism there is an urgent need for the development of new antifungal agents. The role of metal ions is stressed in many important processes and inorganic pharmacology has started to be a significant field with more than twentyfive inorganic compounds being used in therapy as antibacterial, antiviral and anticancer drugs. Recently, metal complexes of biomolecules have been used to design novel antibacterial/antiviral therapies targeted against, for example, human immunodeficiency (HIV) and human papilloma virus (HPV) infections. The hydrazides and their analogues are known to have several different biological activities such as tuberculostatic activity Citation3-5, antibacterial activity [Citation6,Citation7], antifungal activity [Citation8,Citation9], monoamine oxidase inhibitory activity Citation10-12, and antileishmanial activity [Citation13].
Earlier, vanadium complexes with Schiff bases were reported to exhibit a range of biological activities including tuberculostatic activity, antibacterial activity and antifungal activity [Citation14]. Over the past decade, numerous reports have been published on the insulin mimetic properties of vanadate and vanadyl both in in vitro and vivo environments Citation15-18. In view of the various important biological activities of hydrazides and vanadium complexes we envisaged that a combination of hydrazide and vanadium within the same molecule i.e. complex, the activities of hydrazides may be enhanced.
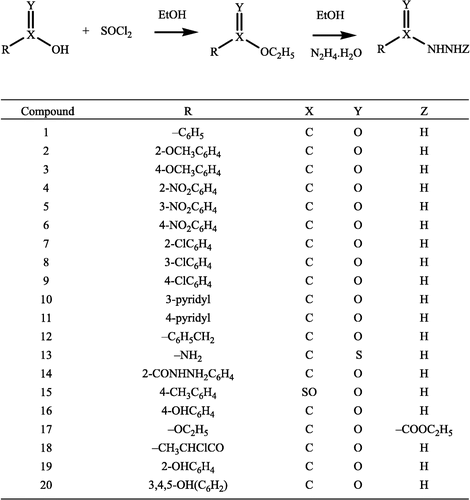
The present report describes a novel series of oxovanadium(IV) hydrazide complexes to explore their antifungal potential. Twenty hydrazides 1–20 alongwith twelve VO(IV) complexes (1a–12a) were synthesized. It was found that ligands 1–20 and complexes 1a–12a showed varying degrees of antifungal activity against A. flavus, T. longifusus, C. albicans, M. canis, F. solani and C. glaberata fungal strains.
Material and methods
All the hydrazides were synthesized according to the literature [Citation13] and were crystallized by methanol. Vanadyl sulphate penta hydrate (VOSO4·5H2O), solvents and all reagents were of analytical grade and used without purification. IR spectroscopic analyses were carried out on a Shimadzu-IR-460 spectrometer in KBr and values are reported in cm− 1. Elemental analyses was carried out on a Perkin Elmer 2400 CHN elemental analyzer. Electronic spectra were recorded on a Shimadzu-UV-1601 spectrophotometer in MeOH and DMSO solution. Magnetic measurements were made on powders by employing a Sherwood-manway-MSB Mk1 magnetic susceptibility balance using sealed off MnCl2 solution as calibrant. Metal contents were determined by a iodometric titration and also confirmed using a 3100 Perkin Elmer Atomic absorption spectrophotometer. Sulphate contents were determined by a gravimetric method and analyzed as BaSO4. Melting points were recorded on a SMP 10 Bibby Stuart Scientific apparatus and are uncorrected.
General method for the preparation of ligands (1–20)
Ethyl-4-nitrobenzoate (5.0 g, 25 mmol) was dissolved in ethanol (75 mL), and then hydrazine hydrate (5 mL, 100 mmol) was added and the mixture refluxed for 5 h. The solid obtained was washed with hexane to afford the hydrazide. Other ligands were prepared from their respective esters.
General method for the preparation of metal(IV) complexes (1a–12a)
A solution of VOSO4·5H2O (1.265 g, 5 mmol) in methanol (10 mL) was added slowly with stirring to a hot methanolic solution (15 mL) of 4-nitrobenzohydrazide (0.905 g, 5 mmol) and the reaction mixture refluxed on a water bath for 2 h, during which time the solid complex separated out. The reaction mixture was cooled to room temperature and the solid complex was filtered and washed with methanol to remove the unreacted metal salt and ligand. The product was dried over anhydrous CaCl2.
Antifungal assay
All the synthesized ligands (1–20) and their respective metal VO(IV) chelates (1a–12a) were screened for in vitro antifungal activity against A. flavus, T. longifusus, C. albicans, M. canis, F. solani and C. glaberata using the agar tube dilution assay Citation19-21. Test samples were dissolved in sterile DMSO to serve as stock solution. Sabouraud dextrose agar was prepared by mixing Sabouraud 4% glucose agar and agar agar in distilled water. It was then stirred with a magnetic stirrer for dissolution and a known amount was dispensed into screw capped test tubes. Test tubes containing media were autoclaved at 121°C for 15 min. Tubes were allowed to cool to 50°C and the test samples of the desired concentrations were taken from the stock solution into the non-solidified Sabouraud agar media. Tubes were then allowed to solidify in a slanting position at room temperature. Each tube was inoculated with a 4 mm diameter piece of inoculum removed from a seven day old fungi culture. All culture containing tubes were inoculated at an optimum temperature of 28–30°C for growth for 7–10 days. Humidity (40% to 50%) was controlled by placing an open pan of water in the incubator. Cultures were examined at least twice weekly during the incubation. After incubation for 7–10 days, the test tubes with no visible growth of the microorganism were taken to represent the minimum inhibitory concentration (MIC) of the test samples, which were expressed in μg/mL. Miconazole and amphotericin B were used as standard drugs.
Results and discussion
Chemistry
The ligands (1–20) were prepared by refluxing hydrazine hydrate with the corresponding esters in ethanol (Scheme ). The structures of these synthesized ligands were determined by spectroscopy and micro analytical data (). All metal complexes of these ligands were prepared in same equimolar ratio.
Table I. Physical, spectral and analytical data of ligands (1–20).
In the solid state all the complexes are fairly stable in air so as to allow physical measurements. These complexes are very soluble in DMF and DMSO but insoluble in common organic solvents, are amorphous solids and have melting points >300°C. Elemental analysis and physical data () for the complexes indicate that the reaction of the ligand with VOSO4·5H2O yielded binuclear complexes in which two metals are coordinated with two ligands while bridging through oxygen atoms of hydrazides. Spectral data for the metal chelates are presented in .
Table II. Physical and analytical data of metal complexes.
Table III. Spectral data of the metal chelates.
Biology
All the synthesized ligands 1–20 and complexes 1a–12a were screened for their antifungal activities and the results are shown in . The compounds were tested against six fungal strains, namely Aspergillus flavus (A. flavus), Trichophyton longifusus (T. longifusus), Candida albicans (C. albicans), Microsporum canis(M. canis), Fusarium solani (F. solani) and Candida glaberata (C. glaberata). The results were compared with the standard drugs miconazole and amphotericin. Compounds which showed 80% or above growth inhibition were selected for minimum inhibitory concentration (MIC) studies. Compounds 1a, 2, 2a, 3, 4a, 5, 5a, 6, 6a, 7, 7a, 8a, 10, 11, 11a, 12a, 13, 14, 15 and 16 were selected for MIC against the respective strains. Compounds showed varying degrees of activity against almost all the tested fungi.
Table IV. Results of antifungal bioassay (% inhibition).
The present investigation indicates that most of the hydrazides 3, 5, 7, 8, 13, 15, 16, 17 along with their oxovanadium(IV) complexes 3a, 5a, 7a, 8a showed more significant antifungal activity against M. canis. The screening test showed that compound 4, 9, 10, 11 and 12 are potent against M. canis while their oxovanadium(IV) complexes 4a, 9a, 10a, 11a and 12a are inactive against this fungal strain.
It is revealed from the data that some hydrazides 2, 3, 5, 6, 7 showed strong growth inhibitory activity against A. flavus but their complexes 2a, 3a, 5a, 6a and 7a were found to be very weakly active or inactive against this fungal strain. In contrast some complexes 1a, 4a, 11a, 12a showed significant activity but their ligands 1, 4, 11, 12 are inactive against A. flavus. From the present studies it is observed that compound 3a, 4, 5, 5a, 7, 8a and 9, 13 and 14 displayed good antifungal profile against T. longifusus.
Compounds 1, 9a, 10a, 18–20 were found completely inactive against all the tested fungal strains.
Notably, some fungi, C. albicans, F. solani and C. glaberata were almost resistant to all tested compounds except compound 9 which exhibit 50% inhibition against F. solani.
The minimum inhibitory concentration (MIC) of eleven ligands and nine vanadium(IV) complexes were determined using the agar tube dilution assay Citation19-21. The results are tabulated in . The compounds were tested against three fungal strains, namely A. flavus, T. longifusus and M. canis. The MIC range of these selected vanadium compounds varied from 250–400 μg/mL (188–671 μM). In contrast, most of the ligands showed low inhibitory actvity. The compounds 1a, 2, 3, 4a, 6, 7, 11a, 12a, and 14 have significant potential against A. flavus, whereas compounds 5, 7, 13 and 14 are active against T. longifusus. Some compounds 2a, 3, 5a, 6a, 7a, 8a, 10, 11, 13, 15 and 16 displayed a better antifungal profile against M. canis.
Table V. Minimum inhibitory concentration (μg/mL).
Acknowledgement
The authors are extremely grateful to the Higher Education Commission (H.E.C.) for granting financial assistance for these studies.
Notes
*Maqsood, Z. T. et al., manuscript in preparation with detailed chemistry.
References
- Albert A. Aust J Sci 1967; 30(1), [Chem Abstr 68, 20603f]
- Krischner S, Wei YK, Francis D, Bergman JG. J Med Chem 1966; 9: 369
- Buu-Hoï NP, Xuong ND, Nam NH, Binon F, Royer R. J Chem Soc 1953; 1358
- Waisser K, Odlerova Z, Houngbedji N, Thiel W, Mayer R. Zentralbl Mikrobial 1989; 144: 355
- Waisser K, Houngbedji N, Odlerova Z, Thiel W, Mayer R. Pharmazie 1990; 45: 141
- Ciugureanu C, Ungureanu M, Grosu G. Rev Med Chir 1993; 97(433), [Chem Abstr 123, 52102n]
- Fukujiro F, Kunio N, Akimasa T, Yuhei H, Tokugoro O, Takeya H, Motohisa F. J Pharm Soc Jpn 1954; 74: 884
- Schering AG. Neth Appl 1967; 6: 612–874, [Chem Abstr 68, 59334u]
- Thu-Cuc NT, Buu-Hoï NP, Xuong ND. J Med Pharm Chem 1961; 3(361), [Chem Abstr (1961) 15398g]
- Hall LH, Mohney BK, Kier LB. Quant Struct Act Relat 1993; 12(44), [Chem Abstr 120, 48683y]
- Cardellini M, Claudi F, Grifantini M, Gulini U, Martelli S. J Pharm Sci 1977; 66: 259
- Safrazbekyan RR, Sukasyan RS, Arzanunts EM. Vopr Med Khim 1979; 25(311), [Chem Abstr 91, 69259n]
- Khan KM, Rasheed M, Zia-Ullah, Hayat S, Kaukab F, Choudhary MI, Atta-ur-Rahman, Perveen S. Bioorg Med Chem 2003; 11: 1381, and references quoted therein
- Hashidhara GM, Goudar TR. Synth React Inorg Met-Org Chem 2000; 30: 1581
- Shechter Y, Shisheva A, Lazar R, Libmann J, Shazner A. Biochemistry 1992; 31: 2063
- Posner BL, Shaver A, Fantus IG. New antidiabetic drugs. 1990; 107
- Shechter Y, Meyerovitch J, Farfel Z, Sack J, Bruck R, Amir S, Degani H, Karlish SJD. Vanadium in biological systems. 1990; 4: 129
- Orvig C, Thompson KH, Battell M, McNeill JH. Met Ions Biol Sys 1995; 31: 575
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. Harwood Academic Publishers, The Netherlands 2001; 22
- Blank H, Rewbell G. Arch Dermatol 1965; 92: 319
- Brass G, Shainhouse JZ, Stevens DA. Antimicrob Agents Ch. 1979; 15: 763