Abstract
Several coumarin derivatives have been reported to present multiple biological activities and especially anti-inflammatory/antioxidant activities. Recently the synthesis and in vivo/in vitro anti-inflammatory /antioxidant activities of several new coumarin derivatives with a 7-azomethine linkage have been reported. In the present study these derivatives were further tested for their antioxidant ability. Some of them were found in vitro to inhibit lipid peroxidation and to strongly scavenge superoxide radicals. Compound 3 was found to potently inhibit cyclooxygenase-1 (COX-1) and the yeast-induced rat paw oedema. The most active compounds within the set were tested against adjuvant-induced arthritis. Compound 3 was found to significantly protect the rats from adjuvant-induced arthritis (when it is administered from the first day or when it is administered the fourteenth day, with the first symptoms of the disease). An attempt was made to delineate the possible mechanism of action of the studied compounds.
Introduction
Coumarins comprise a large class of phenolic substances occuring in plants [Citation1]. Coumarins, natural and synthetic derivatives, were found to possess significant anti-inflammatory and antioxidant activities and have been used to treat various ailments such as cancer, burns, cardiovascular and rheumatic diseases [Citation2]. The coumarin ring has been shown to possess unique anti-oedema and anti-inflammatory activities. Thus, coumarin derivatives could be particularly effective in the treatment of all high protein oedemas Citation3-5. Many coumarin compounds are recognized as lipoxygenase and cyclooxygenase inhibitors Citation6-8. Coumarin derivatives have also been found to have the ability to scavenge reactive oxygen species (ROS) – free radicals, such as superoxide radicals and to influence processes involving free radical-injury [Citation9,Citation10]. They have also been found to inhibit lipid peroxidation and to possess vasorelaxant activities [Citation11].
Inflammation is a complex phenomenon involving interrelationships between humoral and cellular reactions through a number of inflammatory mediators. It is a usual symptom covering different pathologies and there are still many questions to be answered in order to understand the inflammatory process as well as a need for better-tolerated and more efficient non-steroidal anti-inflammatory drugs. In the pathways of the inflammatory process, the implication of free radicals is particularly important. It has been also reported that anti-inflammatory drugs may be effective in the prevention of free radical mediated damage [Citation1].
We have already reported several coumarin derivatives which have significant anti-inflammatory and antioxidant activities Citation11-13. In connection with our previous work we have recently reported the synthesis of several new coumarin derivatives with a 7-azomethine linkage [Citation14]. These coumarin derivatives (compounds 3–9) are shown in . All these compounds have been tested for their anti-inflammatory and antioxidant activity and most of them were found to be potent agents.
Table I. Lipophilicity values: Theoretically calculated C log Pa; Inhibition % of induced carrageenin rat paw edema CPE (%)b; [Citation14] In vitro Lipid Peroxidation (LP %); % scavenging activity on superoxide radicals (PMS%).
In this study we tested the behavior of the 7-azomethine derivatives, on several mediators of inflammation as well as their effect on several oedemas in order to delineate their possible mechanism of action.
Materials and methods
Nordihydroguairetic acid (NDGA) was purchased from the Aldrich Chemical Co. Milwaukee, WI, (USA). Soybean Lipoxygenase, linoleic acid sodium salt arachidonic acid (AA), NADH, nitrotetrazolium blue (NBT), porcine heme and indomethacin were obtained from Sigma Chemical, Co. (St. Louis, MO, USA). For the in vivo experiments, male and female Fischer-344 rats (180–240 g) were used but pregnant females were excluded. The kit for the COX Activity Assay was purchased from Cayman. N-methylphenazonium-methyl sulfate was purchased from Fluka. Freund's Adjuvant refereed to 0.6 mg desiccated Mycobacterium butyricum was suspended in 0.1 ml liquid paraffin. Baker's Yeast was commercially available. Warfarin was obtained from A. Fragos, Abbott Laboratories, Greece.
Biological assays
In vitro studies
In the in vitro assays each experiment was performed at least in triplicate and the standard deviation was less than 10% of the mean.
In vitro inhibition of cyclooxygenase-1 (COX-1) [Citation15]
Cyclooxygenase (COX) activity was determined by using arachidonic acid (AA) as substrate and N,N,N,N-tetramethylphenylenediamine (TMPD) as co-substrate, as described by Kulmacz and Lands [Citation15]. The reaction mixture (1 ml) contained 0.75 μM heme, 128 μM TMPD, 80 μM AA and 1.5 μg enzyme, in 0.1 M Tris/HCl (pH 8.5). The oxidation of substrate, starter of the reaction, was measured at 37°C by monitoring the increase in absorbance at 611 nm. The absorption due to the spontaneous oxidation of TMPD was subtracted from the initial rate of oxidation observed in the presence of AA. The inhibition of the studied compounds was determined after pre-incubation for 6 min with the enzyme in the presence of heme and TMPD and the reaction was started by adding AA. Sc-560 has been used as a comparative COX-1 inhibitor.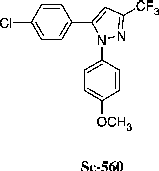
Heme protein-dependent lipid degradation [Citation16]
50 μM heme, arachidonic acid (0.4 mM), the compounds at the various concentrations tested, and H2O2 (0.5 mM) were incubated together for 10 min at 37°C in KH2PO4–KOH buffer (50 mM, pH 7.4). The product of peroxidation was detected using the TBA test [Citation17]. The compounds were added in DMSO solution, which has no effect on the assay.
Non–enzymatic assay of superoxide anion radicals – measurement of superoxide radical scavenging activity [Citation18]
The superoxide producing system was set up by mixing phenazine methosulfate (PMS), NADH and air – oxygen. The production of superoxide was estimated by the nitroblue tetrazolium method. The reaction mixture containing 3 μM PMS, 78 μM NADH, and 25 μM NBT in 19 μM phosphate buffer pH 7.4 was incubated for 2 min at room temperature and the absorption measured at 600 nm against a blank containing PMS. The tested compounds were preincubated for 2 min before adding NADH.
In vivo studies
These studies were in accordance with recognized guidelines on animal experimentation (Guidelines for the care and use of laboratory animals published by the Greek Goverment 160/1991, based on EU regulations 86/609).
Toxicity of the examined compounds [Citation14]
Toxicity experiments were carried out using both male and female Fischer 344 rats but pregnant females were excluded. The tested compounds were dissolved in water (salts) or suspended with few drops of Tween 80 and ground in water and administered by intra-peritoneal injection at various concentrations. Mortality was recorded after 24 h.
Inhibition of carrageenin induced oedema [Citation11]
Groups of 5 rats weighing 180–220 g were used. A single dose of 0.01 mmol/kg body weight of the tested compounds, suspended in water with a few drops of Tween 80 and ground in a mortar before use, was given intra-peritoneal ip at the same time as the carrageenin. Carrageenin (2%) 0.1 ml was injected intra-dermal into the right foot pad, the left paw serving as a control. Indomethacin (reference drug) was administered intra-peritoneal simultaneously with the administration of the phlogistic agent. Controls received the liquid vehicle. The animals were euthanized 3.5 h after carrageenin injection. Both hind paws were severed above the ankle joint and immediately weighed in a very sensitive analytical balance. The experiment was repeated twice for each compound (two groups of 5 animals). The difference between the weight of the injected and un-injected paws was calculated for each animal. The change in paw weight was compared with that in control animals (injected with water) and expressed as a percent inhibition of the oedema (CPE% values, ).
Inhibition of yeast-induced oedema [Citation19]
Oedema was induced in the right hind paw of Fisher 344 rats (150–200 g) by the intradermal injection of 0.1 ml 5% baker's dry yeast in water. Both sexes were used but pregnant females were excluded. Each group was composed of 6–15 animals. The animals, which had been bred in our laboratory, were housed under standard conditions and received a diet of commercial food pellets and water ad libitum during maintenance but were entirely fasted during the experimental period. Our studies were in accordance with recognised guidelines on animal experimentation.
The tested compound 3 at 0.01 mmol/kg body weight was suspended in water, with a few drops of Tween 80 and ground in a mortar before use and was given ip simultaneously. The rats were euthanized 3.5 h after the baker's yeast injection. The difference between the weight of the injected and un-injected paws was calculated for each animal. The change in paw weight was compared with that in control animals (treated with water) and expressed as a percent inhibition of the oedema (YPE% values, ). Indomethacin was used as a reference drug. YPE% values are the mean from two different experiments with a standard error of the mean less than 10%.
Table II. In vitro inhibition of cyclooxygenase (COX%) and in vivo inhibition of yeast-induced paw edema (YPE %)a.
Induction of adjuvant induced disease (AID)[Citation14]
Preventive test
Groups of 5 animals were used. Rats were divided into 3 groups: groups 1 and 2 were injected [Citation19] with Freund's adjuvant (FA) intra-dermal, into the base of the tails of the animals and were treated with compounds 7-hydroxy-coumarin, 3, 5 and 6, which were found to be the most active in the carrageenin paw oedema test [Citation14]. Compounds were injected i.p. in a dose of 0.001 mmol/ kg from day zero once every other day for the following 24 days. Group 3 used as an absolute control, was injected with liquid vehicle only. Adjuvant arthritis was developed about 14 days post FA administration. Arthritic score was measured every 2 days from the commencement (14th day). For quantification of arthritis (arthritic score), a single point was assigned for each inflamed wrist or ankle area and an additional point was given for each involved phalange joint, up to a maximum of 5 points per extremity (). On the 24th day and at least 12 h after the last injection, animals were administered zoxazolamine i.p 10 mg/ 1 ml/g, as an aqueous suspension with a few drops of Tween 80 and the duration of the paralysis and the inflammation were assessed on the 24th day (). The experiment was conducted in duplicate.
Table IIIa. Effect of compounds 7-hydroxy-coumarin, 3, 5, 6 on the onset (day of appearance after Adjuvant's injection, FA) and severity of arthritis (arthritic scores) in Adjuvant Induced Disease (AID) rats.
Table IIIb. Assessment of the preventive action of 7-hydroxy-coumarin and of compounds 3, 5 and 6, on the Adjuvant Induced Disease (AID) manifestations (body weight change, inflammation-arthritic score, zoxazolamine paralysis).
Therapeutic test
The same procedure as above was followed. Adjuvant arthritis was developed about 14 days post FA administration. Compound 3 was injected i.p. in a dose of 0.001 mmol/ kg from day 14 (commencement day) once every other day for the following 10 days. Arthritic score was measured every 2 days from the commencement (14th day). On the 24th day and at least 12 h after the last injection, animals were administered zoxazolamine i.p 10 mg/1 ml/g, as an aqueous suspension with a few drops of Tween 80 and the duration of the paralysis and the inflammation were assessed on the 24th day (,). The experiment was conducted in duplicate.
Table IVa. Therapeutic effect of compound 3 on the onset (day of appearance after Adjuvant's injection, FA) and severity of arthritis (arthritic scores) in Adjuvant Induced Disease (AID) rats. Rats were treated with compound 3 after the appearance of arthritis.
Results and discussion
In some steps of the inflammatory process, the implication of free radicals is particularly important, one of the strongest arguments in favor of this being the beneficial effect of superoxide desmutase (SOD) especially in joint inflammation [Citation20].
It has been reported that anti-inflammatory drugs may also be effective in the prevention of free radical mediated damage [Citation21]. It is therefore to be considered that the action of some anti-inflammatory agents may be due to their antioxidant and free radical scavenging properties.
We have previously reported the in vivo anti-inflammatory activity (inhibition of carrageenin rat edema) and the in vitro antioxidant effects (DPPH interaction, √OH radical scavenging activity) of some new coumarin derivatives [Citation14]. Compounds 1, 3, 6 and 7 () demonstrated good anti-inflammatory activity in the carrageenin rat paw edema (54.0–58.6%) [Citation15]. The differences in activity were only quantitative among the members of the series, the oxime being more potent.
In order to clarify the ability of our compounds to inhibit oxidative damage we undertook a characterization of their antioxidant activity in vitro. Biologically relevant reactive oxygen species and assays were used. Mixing heme proteins with H2O2 generates powerful oxidizing, activated heme species and radicals on amino acid side-chains that can cause lipid peroxidation [Citation22]. As a model of such reactions we used the peroxidation of arachidonic acid by a mixture of hemoglobin and H2O2 [Citation23].
In this investigation compounds 4, 7, 8 and 9 were shown to significantly inhibit lipid peroxidation; 4 was the most potent. For compounds 4, 7 and 9 the IC50 values were determined. Compound 2 and unexpectedly compounds 3 and 6 gave low inhibition (mean value c. 5%) whereas compound 5 was found inactive. Compound 8 was gave mild activity (29%).
There is an agreement between anti-inflammatory and antioxidant activity (compounds 4 and 7). It could indicate that organic peroxy-radicals, such as lipoperoxy-radicals, can be scavenged by these compounds and this may be implicated in the mechanism of their anti-inflammatory ability. Furthermore, other antioxidants have been reported to possess anti-inflammatory activity [Citation24].
In compound 3 the free OH group was correlated with a dramatic decrease in antioxidant activity, compared to the CH3-substituted oxime 4. It can be concluded that steric effects and lipophilicity (e.g. compounds 5, 6) are mostly important for the antioxidant activity of these compounds. A possible explanation of this could be the greater ability of compounds 4, 7 and 9 compared with compounds 2, 3, 5 and 6 to concentrate within hydrophobic regions, such as the interior of membranes. The tested compounds were found to inhibit significantly the oxidation of DMSO [Citation14]. This inhibition was principally due to their √OH scavenging activity.
Although hydroxyl radicals formed in the body can lead to the generation of carbon-centred and peroxyl radicals [Citation25], direct relationship between √OH and lipid peroxidation has yet to be established. As far as inflammation is concerned it has been suggested that non-steroidal anti-inflammatories blocked superoxide anion radical production but did not work as radical scavengers [Citation26].
On the other hand it has been proposed that HO√ and not O2√ − is the ultimate harmful species since the destructive process in inflammation can be prevented not only by SOD but also by catalase, HO√ scavengers and chelators, which remove transition metals, thus interrupting the Haber–Weiss reaction that leads to HO√. Further more, it has been claimed that compounds acting as antioxidants and O2√ − scavengers could act as cyclooxygenase inhibitors. In order to further investigate the mechanism of action, compound 3 (the most potent derivative in carrageenin rat paw oedema assay) was tested in vitro as a possible cyclooxygenase inhibitor; Compound 3 was found to be very active (78.9%, ).
Adjuvant induced disease (AID) is a good experimental model for rheumatoid arthritis [Citation11], which is therefore often used in testing agents for anti-inflammatory activity. During this experiment the examined points are: inflammation (counted as arthritic scores), drug metabolic impairment (counted as time of zoxazolamine paralysis) and cachexia (change in body weight). Coumarin derivatives 3, 5 and 6 were selected, since they had been found to be amongst the most active of these compounds in vivo/in vitro for the investigation of anti-AID activity. Two different regimens of treatment were used: the tested compounds 3, 5, 6 and 7-hydroxy-coumarin were administered intra-peritoneally on the 1st day, i.e. the day of the administration of Freund's adjuvant (FA) and in a second experiment, the treatment with compound 3 started on the 14th day post-FA administration, when inflammation and other adjuvant arthritis symptoms (drug metabolic impairment and cachexia) had developed. This arrangement was used to verify at which stage of the disease these compounds act and therefore to clarify whether their action was purely therapeutic or whether they have only a preventive action. Arthritic score, body weight loss and in vivo zoxazolamine metabolism impairment (expressed as the duration of the induced paralysis) were significantly reduced in both series of experiments. The time course of adjuvant arthritis development expressed as arthritic score is shown in Tables and . The effect of the tested compounds on inflammation is also shown in Tables and . Rats treated with compounds 3, 5 and 6 either did not develop or developed very mild arthritis and simultaneously indicated anti-inflammatory activity [Citation14].
Table IVb. Assessment of the therapeutic action of compound 3 on the Adjuvant Induced Disease (AID) manifestations (body weight change, inflammation-arthritic score, zoxazolamine paralysis). Rats were treated with compound 3 after the 14th day, when arthritis had appeared.
However, when the treatment started before the development of the signs of AID, the depression of the adjuvant arthritis symptoms was very well expressed, while, when the treatment started after the full development of the disease, this suppression, though significant, was less expressed than that in the case of the early treatment. These results indicate that the tested compounds act better at the early stages of the induction of the disease than in later stages, when arthritis is fully developed. Nevertheless, it can be concluded that the examined compounds possess not only preventive, but also curative activity on AID.
Compound 3 is a coumarin derivative presenting inhibitory effect in carrageenin induced rat paw oedema and curative and preventive effect in AID. The incipient pattern of carrageenin induced oedema is characterized by the participation of histamine and 5-hydroxytryptamine (5-HT). Compound 3 exerts an effect greater than that of indomethacin, which has potent anti-inflammatory activity. The inhibition on incipient oedema induced by carrageenin suggests that this action of 3 may be stronger on inflammation induced by histamine, 5-HT or kinins. To clarify this situation, the effect of compound 3 and indomethacin on yeast-induced oedema was examined. It has been demonstrated [Citation19] that the time course pattern of oedema induced by yeast in rats resembles that of oedema induced by 5-HT alone. Other investigators [Citation19,Citation27] reported that the inhibitory activity effect of indomethacin on yeast-induced oedema induced by 5-HT was weak, diphenylhydramine had no effect, cycloheptadine inhibited markedly and that yeast oedema was induced by the release of 5-HT from mast cells. In contrast to Brewer's yeast-induced oedema in rats, which appears and disappears rapidly Baker's yeast-induced oedema appears rapidly but remains for several hours. shows the effect of a single dose of compound 3 or indomethacin on Baker's yeast-induced oedema. The inhibition observed by 0.01 mmol/kg of compound 3 was greater than that of indomethacin.
Conclusions
In this investigation it has been shown that most of the examined compounds inhibit lipid peroxidation and scavenge free radicals thus exhibiting an antioxidant activity. It has been established that active oxygen species are implicated in various stages of the process of inflammation, e.g. stimulation of phagocytosis, leucotriene biosynthesis. Leukotrienes play a pathogenetic role in autoimmune diseases and we have previously reported [Citation15] that these derivatives are also potent lipoxygenase inhibitors. Thus, the above tested compounds 3, 5 and 6 appear to be effective agents not only on acute but also on chronic inflammation – arthritis, being possibly effective in autoimmune diseases.
References
- Hoult JRS, Paya M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol 1996; 27: 713–722
- Murray RDH, Mendez I, Brawn SA. The natural coumarins. Wiley, New York 1982
- Piller NB. A comparison of the effectiveness of some anti-inflammatory drugs on thermal oedema. Br J Exp Pathol 1975; 56: 554–559
- Piller NB, Casley-Smith JR. The effect of coumarin on protein and PVP clearance from rat legs with various high protein oedemas. Br J Exp Pathol 1975; 56: 439–445
- Fylaktakidou K, Hadjipavlou-Litina D, Litinas K, Nicolaides D. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activity. Curr Pharm Des 2004; 30: 3813–3833
- Neichi T, Koshihara Y, Mutora SI. Inhibitory effect of esculetin on 5-lipoxygenase and leukotriene biosynthesis. Biochim Biophys Acta 1983; 753: 130–132
- Kimura Y, Okuda H, Arichi S, Baba K, Kozawa M. Inhibition of the formation of 5-hydroxy-6,8,11,14-eicosatetraenoic acid from arachidonic acid in polymorphonuclear leukocytes by various coumarins. Biochim Biophys Acta 1985; 834: 224–229
- Craven PA, Pfanstiel J, De Rubertis FR. Role of reactive oxygen in bile salt stimulation of colonic epithelial proliferation. J Clin Invest 1986; 77: 850–859
- Larson RA. Phytochemistry 1988; 27: 969–980
- Mora A, Paya M, Rios JL, Alcaraz MJ. Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem Pharmacol 1990; 40: 793–797
- Kontogiorgis C, Hadjipavlou-Litina D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J Enzyme Inhib Med Chem 2003; 18: 63–69
- Fylaktakidou KC, Gautam D, Hadjipavlou-Litina D, Kontogiorgis C, Litinas K, Nicolaides D. Reactions of 4-methylchromene-2,7,8-trionewith phosphonium ylides. Synthesis and evaluation of fused 1,3-dioxolaneocoumarins as antioxidants and antiinflammatories. J Chem Soc Perkin Trans 2001; 1: 3073–3079
- Nicolaides D, Gautam D, Litinas K, Hadjipavlou-Litina D, Kontogiorgis C. Synthesis and biological evaluation of Benzo[7,8]chromeno[5,6-b][1,4]oxazin-3-ones. J Heterocycl Chem 2004; 41: 605–611
- Kontogiorgis CA, Hadjipavlou-Litina D. Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg Med Chem Lett 2004; 14: 611–614
- Kulmacz RJ, Lands WEM. Cyclooxygenase: measurement, purification and properties. Prostaglandins and related substances: A practical approach, C Benedetto, RG McDonald-Gibson, S Nigam, TF Slater. Irl. Press, Oxford (England) 1987; 209
- Jeding I, Evans PJ, Akanmu D, Dexter D, Spencer DJ, Aruoma IO, Jenner P, Halliwell B. Characterization of the potential antioxidant and pro-oxidant actions of some neuroleptic drugs. Biochem Pharmacol 1995; 49: 359–366
- Evans PJ, Cecchini R, Halliwell B. Oxidative damage to lipids and alpha 1-antiproteinase by phenylbutazone in the presence of heme proteins: protection by ascorbic acid. Biochem Pharmacol 1992; 44: 981–984
- Candan F. Effect of Rhus coriaria L. (Anacardiaceae) on superoxide radical scavenging and xanthine oxidase activity. J Enzyme Inhib Med Chem 2003; 18: 59–62
- Gavalas A, Kourounakis L, Litina D, Kourounakis P. Antiinflammatory and immunomodulating effects of a novel agent (2-aminoethylamino)butylothienone inhibitor effects on mouse paw edema. Arzneim-Forsch/Drug Res 1991; 41(1)423–426
- Roberfroid MB, Viche HG, Remacle J. Advances in drug research, D Testa. Academic Press, London 1987
- Hiller PL, Hodd KO, Wilson RL. Anti-inflammatory drugs: protection of a Bacteria virus as an in vitro biological measure of free radical activity. Chem Biol Interact 1983; 17: 293–305
- Davies MJ. Detection of myoglobin-derived radicals on reaction of metmyoglobin with hydrogen peroxide and other peroxidic compounds. Free Radic Res Commun 1990; 10: 361–370
- Kelder PP, Fischer MJE, de Mal WJ, Janssen LHM. Oxidation of chlorpromazine by mehtyloglobin in the presence of hydrogen peroxide. Formation of chlorpromazine radical cation and its covalent binding to methemoglobin. Arch Biochem Biophys 1991; 284: 313–319
- Bragt PC, Bransberg JL, Banty IL. Antiinflammatory effects of free radical scavengers and antioxidants: further support for proinflammatory roles of endogenous hydrogen peroxide and lipid peroxides. Inflammation 1980; 4: 289–299
- Aruoma OI, Wasil M, Halliwell B, Hoey BM, Butler J. The scavenging of oxidants by sulphasalazine and its metabolites. A possible contribution to their anti-inflammatory effects?. Biochem Pharmacol 1987; 36: 3739–3742
- Miyazawa K, Limori Y, Makino M, Mikami T, Miyasaka K. Effects of some non-steroidal anti-inflammatory drugs and other agents on cyclooxygenase and lipoxygenase activities in some enzyme preparations. Jpn J Pharmacol 1985; 28: 199–205
- Tsurumi K, Kyuki K, Niwa M, Kokuba S, Fujimura H. Pharmacological investigations of the new antiinflammatory agent 2-(10,11-dihydro-10-oxodibenzo[b,f]thiepin-2-yl)propionic acid. 1st communication: inhibitory effects of rat paw edema. Arzneim-Forsch/Drug Res 1986; 36: 1796–1800
- Biobyte Corp. C-QSAR Database 201 West 4th Str., Suite 204, Claremont CA, California 91711, USA.