Abstract
Catalase enzyme (H2O2: oxidoreductase; E.C. 1.11.1.6) was purified from human skin homogenate using ammonium sulfate precipitation and DEAE-Sephadex A50 ion exchange chromatography at 4°C and some characteristics of the enzyme were investigated. The human skin enzyme, having a specific activity of 1354.5 EU/mg proteins was purified with a yield of 43.13% and 1110-fold. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) showed a single band for the enzyme. Inhibition by piroxicam, ketoprofen, diclofenac sodium, sulfamethoxazole and nidazole occurred with I50 values of 0.414, 1.29, 1.8, 3.83, and 8.64 mM, respectively.
Keywords::
Introduction
Catalase (CAT) is a very important enzyme for protection of cells from the toxic effects of H2O2 and radical oxygen species. Superoxide, hydroxyl radical, and hydrogen peroxide reactive oxygen species are generated during metabolism and they attack cell components such as DNA, protein and lipid membrane and sometimes lethal damage may occur. These potentially injurious actions are neutralized by antioxidant enzymes such as catalases, superoxide dismutases, and peroxidases Citation1-6. Superoxide dismutase (which catalyses superoxide anion to H2O2) and catalase are components of an antioxidant complex which are used as a therapeutic for oxidative injury and especially in myocardial ischaemia reperfusion oxidative injury Citation7-9. Catalase catalyzes the decomposition of hydrogen peroxide to molecular oxygen and water without the production of free radicals Citation1-3Citation8.
Although the effects of many drugs on different enzyme activities has been investigated Citation10-16, there have been no any reports on the in vitro effects of piroxicam, ketoprofen, diclofenac sodium, sulfamethoxazole and nidazole on human skin CAT. From a knowledge of the obtained I50 values, potentially undesirable side-effects of widely used drugs can be diminished on CAT activity and body metabolism in therapy.
Methods and materials
Materials
DEAE-Sephadex A50, H2O2, protein assay reagents and chemicals for electrophoresis were purchased from Sigma Chem. Co. All other chemicals used were analytical grade and purchased from either Sigma or Merck.
Measurements of CAT Activity
CAT activity was measured at 25°C according to Beutler's method [Citation17] which depends on the reduction of H2O2 by CAT. The activity measurement was made by monitoring the decrease in absorption at 240 nm due to the reduction of H2O2 at 25°C. One enzyme unit is that involved the reduction of 1 μmol of H2O2 per minute at 25°C, pH 7.5 [Citation17].
Preparation of the Homogenate
Health frozen human skin ( − 25°C) was supplied by the Plastic Surgery Department of Ataturk University Hospital and excess blood, foreign tissue and membranes were removed from the samples. The tissue was suspended in 5 volumes (w/v) of 10 mM phosphate buffer (pH: 7.5) using a mixer at top speed for 3 min. Then, the material was homogenized by an ultrasonic homogenizer for 2 min followed by centrifugation at 7,000 × g for 20 min. The supernatant was used for ammonium sulfate precipitation. Temperature was maintained at 4°C during the homogenization process [Citation2].
Ammonium Sulfate Precipitation and Dialysis
Human skin homogenate was subjected to orderly precipitation with ammonium sulfate (10–20%, 20–30%, 30–40%, 40–50%, 50–60%, and 60–70%). For each respective precipitation, the enzyme activity was determined both in the supernatant and in the precipitate. The enzyme was observed to precipitate at 20–50% ammonium sulfate. The precipitates were dissolved in the minimum volume of 10 mM phosphate buffer (pH 7.5) and dialysed in the same buffer for 2 h with two changes of buffer [Citation2].
Preparation of Ion Exchange Chromatography Material
Ion exchange chromatographic material was prepared from DEAE-Sephadex A50. Ten gram dried DEAE-Sephadex A50 gel was used for 50 ml column (30 × 3 cm) volume. The gel was heated with distilled water at the 80–90°C, to remove foreign bodies and eliminate air. The gel was suspended in 10 mM phosphate buffer (pH 7.5), then packed in a column (3 × 30 cm) and equilibrated and washed with the same buffer. The flow rates for washing and equilibration were adjusted by a peristaltic pump to 50 ml/h [Citation1,Citation2,Citation18].
Purification of Catalase by Ion Exchange Chromatography
A dialyzed and filtered sample was loaded on the DEAE-Sephadex A50 column and the gel was washed with 10 mM phosphate buffer (pH 7.5) until the absorbance of the column eluate at 280 nm is < 0.05. Bound proteins were eluted with a gradient of 0–400 mM sodium chloride in 10 mM phosphate buffer (pH 7.5) at a flow rate of 20 ml/h. Eluates were collected in 2 ml tubes and their respective activity and absorbance were separately determined at 240 nm and 280 nm respectively. Active fractions were collected. All of the procedures were performed at 4°C [Citation1,Citation2,Citation18].
Protein Determination
Quantitative protein determination was spectrophotometrically measured at 595 nm according to Bradford's method with bovine serum albumin as standard [Citation19]. For the optimal pH determination, the enzyme activity was measured in 50 mM Tris-HCl and phosphate buffers within the pH range 7.0–9.0 and 5.0–8.0, respectively.
Sds Polyacrylamide Gel Electrophoresis (Sds-page)
Enzyme purity was determined by Laemmli's procedure using 3% and 8% acrylamide concentrations for running and stacking gel, respectively. To the gel solution was added 10% SDS. The gel was stabilized in a solution containing 50% propanol +10% TCA +40% distilled water for 30 min. The staining was made for about 2 h by a solution of 0.1% Coommassie Brillant Blue R-250+50% methanol +10% acetic acid. Finally, the gel was washed with a solution of 50% methanol +10% acetic acid +40% distilled water until the protein bands were clear [Citation20].
Inhibitor Studies
In order to determine I50 values, activities were determined using a 20 mM constant substrate (H2O2) and different inhibitor concentrations Drugless cuvette activity was taken as 100%. Regression analysis graphs were drawn using % inhibition values by a statistical package (SPSS-for windows; version 10.0). The inhibitor concentrations causing 50% inhibition (I50) were determined from the graphs.
Results
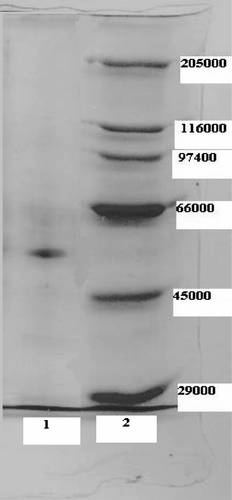
Human skin CAT was purified 1110-fold in a yield of 43.13% by using ammonium sulfate precipitation and DEAE-Sephadex A50 ion Exchange Chromatography (). SDS polyacrylamide gel electrophoresis was performed after the purification of the enzyme, and the electrophoretic pattern was photographed ().
Table I. Scheme for Purification of catalase from human skin.
Figure 1 SDS-PAGE bands of G6PD Lane 1: Human skin CAT. Lane 2: Standards: Rabbit myosin (205,000), E.Coli (-galactosidase (116,000), rabbit phosphorylase B (97,400), bovine albumin (66,000), chicken ovalbumin (45,000), and bovine carbonic anhydrase (29,000)).
In addition, [Drug] vs. % activity graphs were drawn for the tested drugs and are shown in and the I50 values were calculated from and are given in ().
Figure 2 % Activity vs drug concentration regression analysis graphs for human skin CAT in the presence of 5 different concentrations of, (A) piroxicam, (B) Retoprofen, (C) diclofenac, (D) sulfamethoxazole, (E) nidazole.
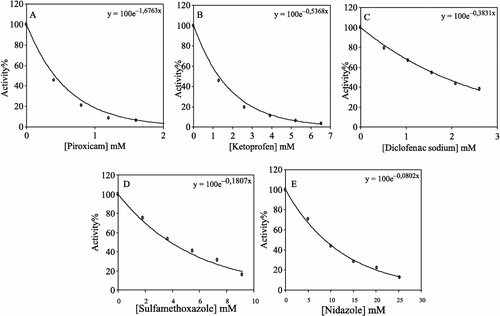
Table II. I50 values for test drugs
Discussion
The importance of CAT in metabolism has been well known for many years. In living cells, biological antioxidant systems are enzymes such as superoxide dismutases (SOD), catalase (CAT), ascorbate peroxidases (APx), glutathione peroxidases (GPx) and glutathione reductase (GR) and non-enzymic components (reduced glutathione (GSH), cysteine, hydroquinones, mannitol, vitamins C and E, flavonoids, some alkaloids and β-carotene. Biological antioxidants can prevent the uncontrolled formation of free radicals and activated oxygen species or inhibit their reactions with biological structures. The destruction of most free radicals and activated oxygen species relies on the oxidation of endogenous antioxidants, mainly scavenging and reducing molecules Citation21-23.
Therefore, in the present study, investigation of effects of some drugs on human skin catalase enzyme was proposed. The catalase enzyme required for this study was purified from human skin by a method consisting of three steps; homogenation, ammonium sulfate precipitation and DEAE-Sephadex A-50.
Piroxicam and ketoprofen are nonsteroidal anti-inflammatory drug effective in treating fever, pain, and inflammation in the body (rheumatoid arthritis). Diclofenac sodium is an anti-inflammatory analgesic often used to treat inflammation and the associated pain, sulfamethoxazole is most commonly used to treat urinary tract infections and omnidazole is active against protozoa and anaerobic bacteria [Citation24].
The human skin catalase was purified by 1110-fold in a yield of 43.13% by using ammonium sulfate precipitation and DEAE-Sephadex A50 ion Exchange Chromatography. SDS polyacrylamide gel electrophoresis was performed after the purification of the enzyme and showed a single band () with Mrc 50, 000.
As shown in the , the obtained I50 values for piroxicam, ketoprofen, diclofenac sodium, sulfamethoxazole and nidazole were 0.414, 1.29, 1.8, 3.83, and 8.64 mM respectively. As evident from the I50 values, catalase inhibition by piroxicam was greater than that observed for ketoprofen, diclofenac sodium, sulfamethoxazole and nidazole.
Average plasma levels for piroxicam, ketoprofen, diclofenac sodium, sulfamethoxazole and nidazole and 0.025, 0.393, 0.006, 0.592, and 0.1168 mM respectively [Citation24]. Accordingly, at these plasma levels the obtained % inhibition by piroxicam, ketoprofen, diclofenac sodium, sulfamethoxazole and nidazole would be 4.2%, 19%, 1%, 11%, and 1% respectively. Therefore as is apparent from these results, catalase inhibition by ketoprofen in vivo could be higher than that observed for sulfamethoxazole, piroxicam, diclofenac sodium and nidazole.
For this reason, if ketoprofen and sulfamethoxazole are given to patients their dosages should be carefully selected to prevent their side-effects on the catalase enzyme and reduce damage to the health of the patient.
References
- Aydemir T, Kuru K, Turk J. Chem 2003; 27: 85–97
- Jang MJ, Park PJ, Jung WK, Kım SK. J Food Biochem 2004; 28: 435–448
- Lemberg R, Legge JW. Hematin compounds and bile pigments. Interscience, New York 1949; 415–424
- Brown-Peterson NJ, Salin ML. J Bacteriol 1993; 175: 4197–4202
- Fridovich I. Arch Biochem Biophys 1986; 274: 1–11
- DeDuve C, Baudhum P. Physiol Rev 1966; 46: 323–357
- Goncalves VM, Leite LCC, Raw I, Cabrera-Crespo J. Biotechnol Appl Biochem 1999; 29: 73–77
- Greenwald RA. Free Radicals Biol. Med 1990; 8: 201–209
- Zughaib ME, Tang XL, Sun JZ, Bolli R, Ann NY. Acad Sci 1994; 723: 218–228
- Honjo T, Watanabe A. Jpn J Antibiot 1984; 37: 32
- Ciftci M, Kufrevioglu OI, Gundogdu M, Ozmen I. Pharmacol Res 2000; 41: 109–113
- Ciftci M, Ozmen I, Buyukokuroglu ME, Penge S, Kufrevioglu OI. Clin Biochem 2001; 34: 297–302
- Ciltas A, Erdogan O, Hisar O, Ciftci M, Israel J. Aquaculture–Bamidgeh. 2003; 55(3)187–196
- Erdogan O, Ciftci M, Çiltas A, Hisar O, Turk J. Vet Anim Sci 1792
- Pickering LK, O'Connor DM, Anderson D, Bairan AC, Feigin RD, Cherry JD. J Infect Dis 1973; 128: 407
- Turck M, Clark RA, Beaty HN, Holmes KK, Karney WW, Reller LB. J Infect Dis 1973; 128: 382
- Beutler E. Red. Academic Press, London 1971; 68–70
- Çoban A, Çiftçi M, Küfrevioğlu Öİ. Prep Biochem Biotech 2002, 2: 173–187
- Bradford MM. Anal Biochem 1976; 72: 248–251
- Laemmli DK. Nature. 1970; 227: 680–683, (Lond)
- Scandalios JG. Plant Physiol 1993; 101: 7–12
- Allen RD. Plant Physiol 1995; 107: 1049–1054
- Chaudiere J, Ferrai-Iliou R. Food Chem Toxicol 1999; 37: 949–962
- Kayaalp SO. Rasyonal tedavi yonunden tibbi farmakoloji, Ankara, Hacettepe-Tas 2002.Yayincilik (Turkish).