Abstract
The inhibitory effects of alkyl phenyl ketones on carbonyl reductase activity were examined in pig heart. In this study, carbonyl reductase activity was estimated as the ability to reduce 4-benzoylpyridine to S( − )-α-phenyl-4-pyridylmethanol in the cytosolic fraction from pig heart (pig heart cytosol). The order of their inhibitory potencies was hexanophenone>valerophenone>heptanophenone>butyrophenone>propiophenone. The inhibitory potencies of acetophenone and nonanophenone were much lower. A significant relationship was observed between Vmax/Km values for the reduction of alkyl phenyl ketones and their inhibitory potencies for carbonyl reductase activity in pig heart cytosol. Furthermore, hexanophenone was a competitive inhibitor for the enzyme activity. These results indicate that several alkyl phenyl ketones including hexanophenone inhibit carbonyl reductase activity in pig heart cytosol, by acting as substrate inhibitors.
Introduction
A variety of xenobiotic compounds contain a ketone or aldehyde group within their chemical structures. These carbonyl compounds are metabolized to the corresponding alcohols in biological systems. Carbonyl reductase (EC 1.1.1.184) is an enzyme that catalyzes NADPH-dependent reduction of carbonyl compounds Citation1-5. Monomeric and tetrameric carbonyl reductases have been purified from the liver, kidney, lung, brain and testis of mammalian species Citation6-11. We have recently purified a tetrameric carbonyl reductase from the cytosolic fraction of pig heart [Citation12]. The purified pig heart carbonyl reductase (PHCR) efficiently reduces alkyl phenyl ketones such as hexanophenone and valerophenone, and α-dicarbonyl compounds such as 9,10-phenanthrenequinone and isatin. Interestingly, PHCR also catalyzes the reduction of all-trans retinal to all-trans retinol, suggesting that it can play a role in retinoid metabolism.
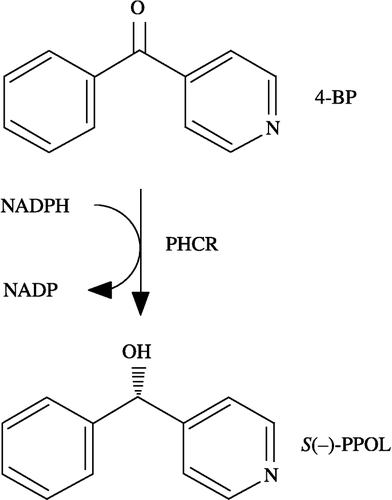
It has been reported that PHCR reduces mainly 4-benzoylpyridine (4-BP) to S( − )-α-phenyl-4-pyridylmethanol [S( − )-PPOL], as shown in [Citation13]. We have also demonstrated that the enantiomeric excess of S( − )-PPOL (89.2% ee) produced in the cytosolic fraction from pig heart (pig heart cytosol) is almost the same as that of S( − )-PPOL (87.2% ee) produced in the reaction system of recombinant PHCR [Citation13,Citation14]. Thus, it is reasonable to assume that most of 4-BP reduction in pig heart cytosol is catalyzed by PHCR.
The substrate specificities of carbonyl reductase for various ketone and aldehyde compounds have been demonstrated. However, information on the substrate inhibition of carbonyl reductase by ketone and aldehyde compounds has been very limited. The purpose of the present study was to elucidate a possible mechanism for the substrate inhibition of carbonyl reductase activity in pig heart by a series of alkyl phenyl ketones. The enzyme (carbonyl reductase) activity was estimated as the ability to reduce 4-BP to S( − )-PPOL, by using pig heart cytosol as the reaction system of PHCR.
Materials and methods
Materials
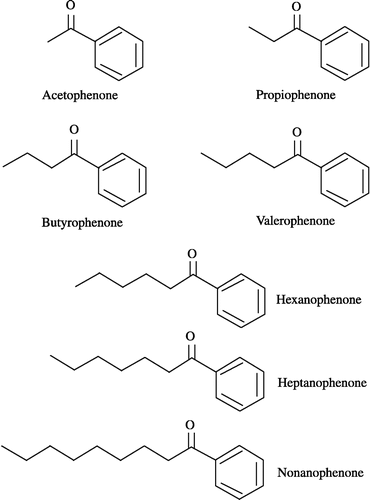
4-BP was purchased from Wako Pure Chemicals (Osaka, Japan). S( − )- and R(+)-PPOL were synthesized from 4-BP as reported previously [Citation13]. All alkyl phenyl ketones () were obtained from Aldrich (Milwaukee, WI) except nonanophenone (Wako Pure Chemicals). All other chemicals were of reagent grade.
Preparation of pig heart cytosol
Pig hearts were supplied from a slaughterhouse and stored at − 20°C. The tissues were homogenized in 3 volumes of 10 mM sodium potassium phosphate buffer containing 1.15% KCl (pH 6.0). The homogenates were centrifuged at 105,000 × g for 60 min to obtain the cytosolic fraction (pig heart cytosol) which was used for the determination of enzyme activity, as described below.
Assay of carbonyl reductase activity
The enzyme (carbonyl reductase) activity was assayed by determining S( − )-PPOL formed from 4-BP in pig heart cytosol. The reaction mixture consisted of substrate (500 μM 4-BP), NADPH-generating system (50 μM NADP+, 1.25 mM glucose-6-phosphate, 50 munits glucose-6-phosphate dehydrogenase and 1.25 mM MgCl2), pig heart cytosol and 100 mM sodium potassium phosphate buffer (pH 6.0) in a final volume of 0.5 mL. The reaction mixture was incubated at 37°C for 10 min and boiled for 2 min to stop the reaction. After centrifugation at 5,000 rpm, the supernatant (20 μL) was subjected to HPLC for the determination of the reduction products [S( − )- and R(+)-PPOL] of 4-BP. HPLC was carried out using a Tosoh DP-8020 HPLC apparatus (Tosoh, Tokyo, Japan) equipped with a Daicel Chiralpak AD-RH column (Daicel, Tokyo, Japan) and a Tosoh UV-8020 monitor (254 nm). A mixture of 20 mM borate buffer (pH 9.0)-acetonitrile (6:4, v/v) was used as a mobile phase at a flow rate of 0.5 mL/min. In the case of inhibition experiments, alkyl phenyl ketones were dissolved in methanol, and added to the reaction mixture at a concentration of 500 μM. The final concentration of methanol did not exceed 2% (v/v), and this concentration did not affect the enzyme reaction. Furthermore, the inhibition of 4-BP reduction (carbonyl reductase activity) by hexanophenone was kinetically analyzed using Lineweaver-Burk plots. Velocity (v) was expressed as nmol/min/mg protein. Protein concentration was determined by the method of Lowry et al. [Citation15] with bovine serum albumin as the standard.
Reduction of alkyl phenyl ketones
The reduction of alkyl phenyl ketones was measured spectrophotometrically by monitoring the decrease in the absorbance of NADPH at 340 nm (ε = 6220 M− 1cm− 1). The reaction mixture consisted of substrate (alkyl phenyl ketone), 0.3 mM NADPH, pig heart cytosol and 100 mM sodium potassium phosphate buffer (pH 6.0) in a final volume of 0.5 mL. The enzyme reaction was initiated by the addition of alkyl phenyl ketones at various concentrations to the reaction mixture. The parameters (Km and Vmax/Km) for the reduction of alkyl phenyl ketones were kinetically determined. One unit of enzyme activity was defined as the amount catalyzing the oxidation of 1 μmol of NADPH/min at 37°C.
Results
Inhibitory effects of alkyl phenyl ketones on carbonyl reductase activity
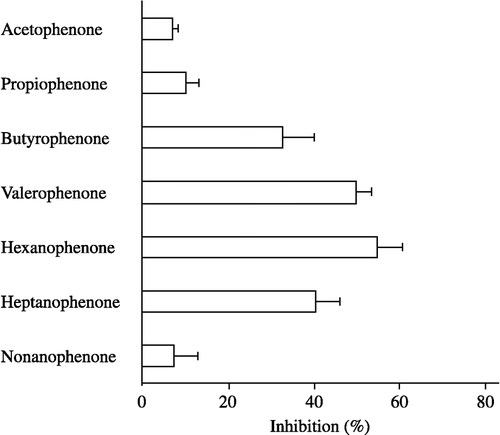
The inhibitory effects of various alkyl phenyl ketones on carbonyl reductase activity were examined in pig heart cytosol, using 4-BP as substrate. As shown in , hexanophenone was the most potent inhibitor for the enzyme activity. Several alkyl phenyl ketones also exhibited significant inhibitions against the enzyme activity. The order of their inhibitory potencies for carbonyl reductase activity was hexanophenone>valerophenone>heptanophenone>butyrophenone>propiophenone. The inhibitory potencies of acetophenone and nonanophenone were much lower.
Figure 3 Inhibitory effects of alkyl phenyl ketones on carbonyl reductase activity in pig heart cytosol. 4-BP at a concentration of 500 μM was used as the substrate. The concentration of alkyl phenyl ketones added as inhibitor was 500 μM. Each bar represents the mean ± S.D. of three to six experiments.
Kinetic parameters for the reduction of alkyl phenyl ketones
The kinetic parameters (Km and Vmax/Km) for the reduction of alkyl phenyl ketones in pig heart cytosol are summarized in . The kinetic parameters for the reduction of acetophenone and nonanophenone were not determined because of the lower catalytic activities. Of the alkyl phenyl ketones tested, hexanophenone exhibited the highest Vmax/Km value. Furthermore, Vmax/Km values of valerophenone, heptanophenone, butyrophenone and propiophenone decreased with the order of their inhibitory potencies for carbonyl reductase activity.
Table I. Kinetic parameters for the reduction of alkyl phenyl ketones in pig heart cytosol.
Relationship between Vmax/Km values and inhibitory potencies
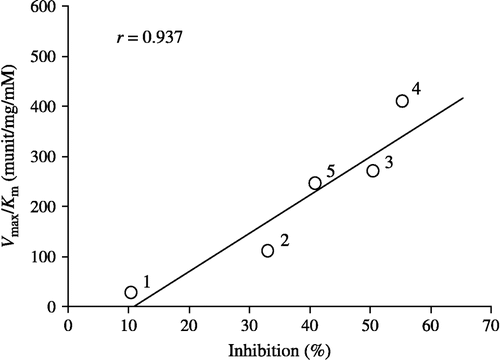
shows the relationship between Vmax/Km values for the reduction of alkyl phenyl ketones and their inhibitory potencies for carbonyl reductase activity in pig heart cytosol. As expected, a significant regression line (r = 0.937, P < 0.05) was obtained from the plots for these alkyl phenyl ketones.
Kinetic mechanism for the inhibition of carbonyl reductase activity by hexanophenone
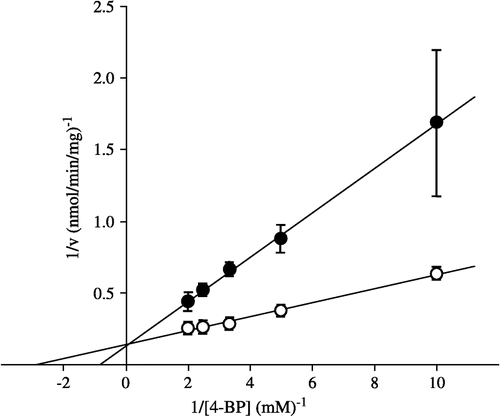
The inhibition of carbonyl reductase activity (the reduction of 4-BP to S( − )-PPOL) by hexanophenone was kinetically examined in pig heart cytosol. As shown in , hexanophenone was found to competitively inhibit the enzyme activity.
Discussion
Carbonyl reductases have been shown to be inhibited by various drugs and flavonoids Citation16-18. For example, nonsteroidal anti-inflammatory drugs such as diclofenac sodium, flufenamic acid and indomethacin effectively inhibit carbonyl reductase purified from rabbit liver, using befunolol as a substrate [Citation17]. Quercetin and quercitrin belonging to flavonoids are well-known inhibitors of carbonyl reductases purified from several tissues [Citation7,Citation8,Citation19,Citation20]. In the present study, we found that alkyl phenyl ketones have the ability to inhibit carbonyl reductase activity in pig heart cytosol. Furthermore, the order of their inhibitory potencies for carbonyl reductase activity was hexanophenone>valerophenone>heptanophenone>butyrophenone>propiophenone>acetophenone = nonanophenone.
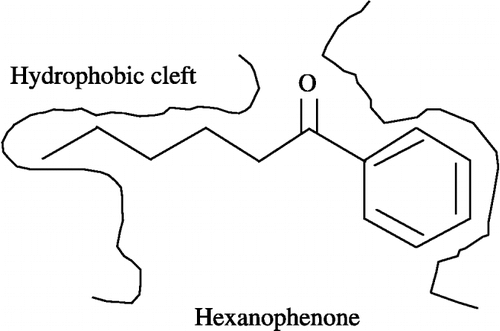
To elucidate a possible mechanism for the substrate inhibition of carbonyl reductase activity by alkyl phenyl ketones, Vmax/Km values for the reduction of alkyl phenyl ketones were further determined. Hexanophenone, valerophenone, heptanophenone, butyrophenone and propiophenone have a straight-chain alkyl group of five, four, six, three and two carbon atoms, respectively, within their chemical structures (see ). Of the alkyl phenyl ketones tested, hexanophenone exhibited the highest Vmax/Km values. In addition, a significant relationship was observed between Vmax/Km values for the reduction of alkyl phenyl ketones and their inhibitory potencies for carbonyl reductase activity in pig heart cytosol. Based on these Vmax/Km values and inhibitory potencies, we propose the possibility that a hydrophobic cleft, which fits most efficiently to a straight-chain alkyl group of five carbon atoms, is located in the substrate-binding domain of PHCR present in pig heart cytosol ().
Furthermore, the inhibition of carbonyl reductase activity by hexanophenone was kinetically analyzed in pig heart cytosol. As expected, hexanophenone was confirmed to be a competitive inhibitor for the enzyme. This finding indicates that hexanophenone inhibits enzyme activity by acting as a substrate inhibitor, and also supports the idea that a hydrophobic cleft corresponding to a straight-chain alkyl group of five carbon atoms in length is located in the substrate-binding domain of PHCR present in pig heart cytosol.
The stereochemistry of ketone reduction has been reported for a number of xenobiotics Citation13Citation21-26. In this study, carbonyl reductase activity was estimated as the ability to reduce 4-BP to S( − )-PPOL in pig heart cytosol. Hexanophenone at a concentration of 500 μM had the ability (55.3 ± 5.9%) to inhibit the reduction of 4-BP to S( − )-PPOL (carbonyl reductase activity) by acting as a substrate inhibitor, as shown in . The reduction of 4-BP in pig heart cytosol involves, in part, the formation of R(+)-PPOL that is the enantiomer of S( − )-PPOL. Interestingly, hexanophenone at a concentration of 500 μM also exhibited a similar inhibition (51.0 ± 5.9%) against the reduction of 4-BP to R(+)-PPOL.
In conclusion, the present study provides evidence that several alkyl phenyl ketones including hexanophenone inhibit carbonyl reductase activity in pig heart cytosol, by acting as substrate inhibitors. Further studies are in progress to elucidate the detailed mechanism for the substrate inhibition of recombinant PHCR by alkyl phenyl ketones and α-dicarbonyl compounds.
References
- Jakoby WB, Ziegler DM. The enzymes of detoxication. J Biol Chem 1990; 265: 20715–20718
- Maser E. Xenobiotic carbonyl reduction and physiological steroid oxidoreduction. The pluripotency of several hydroxysteroid dehydrogenases. Biochem Pharmacol 1995; 49: 421–440
- Oppermann UCT, Maser E. Molecular and structural aspects of xenobiotic carbonyl metabolizing enzymes. Role of reductases and dehydrogenases in xenobiotic phase I reactions. Toxicology 2000; 144: 71–90
- Forrest GL, Gonzalez B. Carbonyl reductase. Chem Biol Interact 2000; 129: 21–40
- Matsunaga T, Shintani S, Hara A. Multiplicity of mammalian reductases for xenobiotic carbonyl compounds. Drug Metab Pharmacokinet 2006; 21: 1–18
- Sawada H, Hara A, Nakayama T, Kato F. Reductases for aromatic aldehydes and ketones from rabbit liver. Purification and characterization. J Biochem (Tokyo) 1980; 87: 1153–1165
- Ikeda M, Hattori H, Ohmori S. Properties of NADPH-dependent carbonyl reductases in rat liver cytosol. Biochem Pharmacol 1984; 33: 3957–3961
- Imamura Y, Higuchi T, Nozaki Y, Sugino E, Hibino S, Otagiri M. Purification and properties of carbonyl reductase from rabbit kidney. Arch Biochem Biophys 1993; 300: 570–576
- Nakayama T, Hara A, Sawada H. Purification and characterization of a novel pyrazole-sensitive carbonyl reductase in guinea pig lung. Arch Biochem Biophys 1982; 217: 564–573
- Wermuth B. Purification and properties of an NADPH-dependent carbonyl reductase from human brain. J Biol Chem 1981; 256: 1206–1213, Relationship to prostaglandin 9-ketoreductase and xenobiotic ketone reductase
- Iwata N, Inazu N, Takeo S, Satoh T. Carbonyl reductases from rat testis and vas deferens. Purification, properties and localization. Eur J Biochem 1990; 193: 75–81
- Usami N, Ishikura S, Abe H, Nagano M, Uebuchi M, Kuniyasu A, Otagiri M, Nakayama H, Imamura Y, Hara A. Cloning, expression and tissue distribution of a tetrameric form of pig carbonyl reductase. Chem Biol Interact 2003; 143–144: 353–361
- Shimada H, Fujiki S, Oginuma M, Asakawa M, Okawara T, Kato K, Yamamura S, Akita H, Hara A, Imamura Y. Stereoselective reduction of 4-benzoylpyridine by recombinant pig heart carbonyl reductase. J Mol Catal B Enzym 2003; 23: 29–35
- Shimada H, Oginuma M, Hara A, Imamura Y. 9,10-Phenanthrenequinone, a component of diesel exhaust particles, inhibits the reduction of 4-benzoylpyridine and all-trans-retinal and mediates superoxide formation through its redox cycling in pig heart. Chem Res Toxicol 2004; 17: 1145–1150
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–275
- Felsted RL, Bachur NR. Mammalian carbonyl reductases. Drug Metab Rev 1980; 11: 1–60
- Imamura Y, Nozaki Y, Higuchi T, Otagiri M. Reactivity for prostaglandins and inhibition by nonsteroidal anti-inflammatory drugs of rabbit liver befubolol reductase. Res Commun Chem Pathol Pharmacol 1991; 71: 49–57
- Imamura Y, Migita T, Uriu Y, Otagiri M, Okawara T. Inhibitory effects of flavonoids on rabbit heart carbonyl reductase. J Biochem (Tokyo) 2000; 127: 653–658
- Terada T, Niwase N, Koyama I, Imamura M, Shinagawa K, Toya H, Mizoguchi T. Purification and characterization of carbonyl reductases from bovine liver cytosol and microsomes. The cytosolic enzyme has a novel 3α/17β-hydroxysteroid dehydrogenase activity. Int J Biochem 1993; 25: 1233–1239
- Atalla A, Breyer-Pfaff H, Maser E. Purification and characterization of oxidoreductases-catalyzing carbonyl reduction of the tobacco-specific nitrosamine 4-methylnitrosamine-1-(3-pyridyl)-1-butanone (NNK) in human liver cytosol. Xenobiotica 2000; 30: 755–769
- Hermans JJR, Thijssen HHW. The in vitro ketone reduction of warfarin and analogues. Substrate stereoselectivity, product stereoselectivity and species differences. Biochem Pharmacol 1989; 38: 3365–3370
- Strolin Benedetti M, Pianezzola E, Fraier D, Castelli MG, Dostert P. Stereoselectivity of idarubicin reduction in various animal species and humans. Xenobiotica 1991; 21: 473–480
- Eyles DW, Pond SM. Stereospecific reduction of haloperidol in human tissues. Biochem Pharmacol 1992; 44: 867–871
- Dow J, Berg C. Stereoselectivity of the carbonyl reduction of dolasetron in rats, dogs, and humans. Chirality 1995; 7: 342–348
- Nagamine S, Horisaka E, Fukuyama Y, Maetani K, Matsuzawa R, Iwakawa S, Asada S. Stereoselective reductive metabolism of metyrapone and inhibitory activity of metyrapone metabolites, metyrapol enantiomers, on steroid 11β-hydroxylase in the rat. Biol Pharm Bull 1997; 20: 188–192
- Rosemond MJC, Walsh JS. Human carbonyl reduction pathways and a strategy for their study in vitro. Drug Metab Rev 2004; 36: 335–361