Abstract
Six novel triorganotin(IV) 2-maleimidopropanoato complexes: R3SnOCOCH3(CH)(COCH)2, (R: Me(1), Et(2), n-Pr(3), n-Bu(4), Ph(5), Bz(6) have been synthesized. Their solid-state configuration has been determined by FT IR and 119mSn Mössbauer spectroscopy. The tin(IV) atom is five-coordinated in all the complexes with 2-maleimidopropanoic acid behaving as a monoanionic bidentate ligand coordinating the tin(IV) atom through a chelating or bridging carboxylate group. The solution-state configuration has been elucidated by means of 1H-, 13C- and 119Sn-NMR spectroscopy which assigned a tetrahedron. Elemental analysis and FAB MS data also supported a 1:1 metal to ligand stoichiometry. The title complexes have been screened in vitro for anti-tumour, anti-fungal, anti-leishmanial and urease inhibition activities and displayed promising results.
Introduction
Metal complexes generally and organotin(IV) compounds especially constitute an important class of compounds which find a number of daily life biomedical and commercial applications like wood preservatives, anti-fouling paints, agricultural fungicides etc. Citation1-4. During the past few decades, pharmaceutical properties of organotin(IV) carboxylates have been investigated for their anti-tumour activity Citation5-9. Generally, the toxicity of organotin(IV) complexes is influenced by the structure of the ligand and its ability to coordinate and the order of toxicity is R3SnR′> R2SnR′2> RSnR′3 [Citation10]. Organotin(IV) carboxylates containing biologically active ligands like amino acids and their derivatives have found considerable attention due to significant bioactivity Citation11-19. Recently, we have reported organotin(IV) complexes of different N-protected amino acids, but triorganotin(IV) esters of 2-maleimidopropanoic acid have not yet been reported and deserve detailed investigation Citation19-23. We are of the view that it was of interest to synthesize, characterize and assess the in vitro bio-potential and structure-activity-relationship of such complexes.
Experimental
Materials
2-Aminopropanoic acid, maleic anhydride, triethylamine, trimethyltin(IV) chloride, triethyltin(IV) chloride, tripropyltin(IV) chloride, tributyltin(IV) chloride and triphenyltin(IV) chloride were Sigma or Fluka products of analytical purity and used as such, while tribenzyltin(IV) chloride was prepared according to a reported procedure [Citation24]. Solvents used during this work were dried as reported [Citation25]. Jack bean and Bacillus pasteurii urease were obtained from Sigma–Aldrich.
Instrumentation
Elemental analyses (C, H, N) were performed on a Yanaco high-speed CHN analyzer; antipyrene was used as a reference, while the tin content was estimated according to reported procedures [Citation26]. Uncorrected melting point was taken on a Reichert Thermovar (F. G. Bode Co., Austria).
The FT IR spectra of the pure solid samples were recorded on a Bruker FT IR spectrophotometer TENSOR27 (ZnSe) using OPUS software covering 5000–400 cm− 1.
For Mössbauer measurements, the solid samples were maintained by liquid nitrogen at a temperature of 77.3°K, using a V. G. Micromass 7070 F Cryolid liquid nitrogen cryostat. The multichannel calibration was performed with an enriched iron foil using 57Co–Pd source, while the zero point of the Doppler velocity scale was determined through the absorption spectra of CaSnO3 (119Sn = 0.5 mg cm− 2). The resulting 5 × 105 -count spectra were refined to obtain the isomeric shift, IS (mm s− 1), the nuclear quadrupole splitting QS, ρ (mm s− 1) and the width at half-height of the resonant peaks, Γ (mm s− 1).
1H- and 13C NMR spectra (CDCl3) were recorded on a multinuclear Bruker Biospin AMX 300 MHz, FT NMR spectrometer using 300 and 75 MHz, respectively, at room temperature employing TMS as internal reference. 119Sn NMR spectra in CDCl3 were recorded at 186.50 MHz on a Bruker AMX 500 spectrophotometer using external neat SnMe4 (δ119Sn = 0 ppm). EI mass spectra were recorded using a model MAT 112, Double-Focusing Mass Spectrometer (Finnigan).
Methods
Synthesis of 2-maleimidopropanoic acid
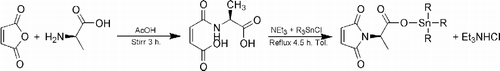
Maleic anhydride (10 g, 101.9782 mmoles) was dissolved in acetic acid (150 mL) and a cold solution of 2-aminopropanoic acid (9.0852 g, 101.9782 mmoles) in acetic acid (150 mL) added. The mixture was stirred at room temperature for 3 h resulting in a white precipitate which was washed thrice with cold water and recrystallized from water to get maleamic acid of analytical purity. Maleamic acid (5 g, 26.7165 mmoles) was suspended in dry toluene (350 mL), triethylamine (7.4806 mL, 53.433 mmoles) added and the suspension refluxed with rigorous stirring for 1.5 h with the concomitant removal of water using a Dean-Stark separator. The solvent was removed on a rotary evaporator (Büchi) leaving a hygroscopic solid; HCl was added up to pH 2 and the residue was extracted with ethyl acetate and the extract dried over anhydrous MgSO4 and evaporated. The solid mass left was recrystallized from hexane (see ).
Synthesis of organotin(IV) complexes of 2-maleimidopropanoic acid
A solution of the triethylammonium salt of 2-maleimidopropanoic acid (0.5 g, 2.9563 mmoles) in dry toluene (75 mL) was prepared and the appropriate amount of triorganotin(IV) chloride (2.9563 mmoles) was added. The mixture was heated to reflux for 4.5 h which resulted in turbidity due to the formation of triethylammonium hydrochloride, which was filtered off and the filtrate was evaporated on a rotary evaporator. The solid mass was dissolved in a mixture of C6H6and C6H14 (1:1) and the compound was recrystallized from CH2Cl2 ().
Partition coefficient, in vitro anti-tumour, anti-leishmanial and anti-fungal activity
Partition coefficient measurement; and the protocol for in vitro anti-tumour, anti-leishmanial and anti-fungal activity is described elsewhere Citation20-23.
Urease assay and inhibition
A reaction mixture containing 10 ul/mL of 20 mg/mL and 2.5 mg/mL, respectively, of Jack bean and Bacillus pasteuri ureases (Sigma, catalog numbers U1500 and U7127, respectively) and 10 μL of 0.01, 0.02, 0.05 and 0.1 mM of test compound (1–7) were incubated at 30°C for 15 min in 96-well plates and then 55 μL of buffers containing 100 mM urea were incubated for 15 min. Then, final urease activity was determined by measuring ammonia production using the indophenol method as described by Weatherburn [Citation27]. Briefly, 45 μL each of phenol reagent (1% w/v phenol and 0.005% w/v sodium nitroprusside) and 70 μL of alkali reagent (0.5% w/v NaOH and 0.1% active chloride NaOCl) were added to each well. The increasing absorbance at 630 nm was measured after 50 min, using a microplate reader (Molecular Device, USA). All the reactions were performed in triplicate in a final volume 200 μL. The results were processed using SoftMax Pro software (Molecular Device, USA). All the assays were performed at pH 8.2 (0.01 M K2HPO43H2O, 1 mM EDTA and 0.01 M LiCl). Percentage inhibitions were calculated from the formula 100 − [(ODtestwell/ODcontrol) × 100]. Thiourea was used as the standard inhibitor of urease.
Results and discussion
The complexes and the ligand were synthesized by the general procedure depicted in . Analytical data for the complexes showed metal/ligand 1:1 stoichiometry. All the compounds were quite stable, obtained in good yield (77–88%) and were soluble in most organic solvents. Elemental analysis data found were in good agreement with calculated data. The physical and analytical data for the investigated compounds are reported in .
Table I. Physical and analytical data for complexes 1–6 and the ligand 7.
Solid state FT IR and 119mSn Mössbauer spectroscopic results
Diagnostically important vibrational bands like ν(COO)asym, ν(COO)sym, ν(Sn–C) and ν(Sn–O) were assigned in the spectral range 1700–400 cm− 1 and are presented in for 1–7. The complexation of the tin(IV) with the ligand was confirmed by the presence of Sn–O and Sn–C bands in the range of 426–451 cm− 1 and 521–540 cm− 1 respectively. In the IR spectra of 7, a broad band at 3200–2800 cm− 1 for –COOH was observed which was absent in the spectra of 1–6. Based on the difference, Δν between ν(COO)sym and ν(COO)asym and the corresponding band position, it is proposed that the carboxylate group acted as a bidentate in all these complexes [Citation28]. It is reported in the literature that Δν values>200 and < 350 cm− 1 reflected the bidentate coordination in chain structures formed by bridging carboxylate groups for 1–6 () [Citation19,Citation21]. Moreover, a characteristic sharp peak at 450 cm− 1 was seen in the spectrum of 5 which also confirmed the Sn–Ph linkage () [Citation29].119mSn Mössbauer parameters (QS, IS, Γ1 and Γ2) are also helpful in the determination of the solid-state configuration of organotin(IV) carboxylates Citation15-18. 119mSn Mössbauer spectra of 1–5 showed quadrupole splitting (QS) value>3.50 mms− 1; literature reveals that triorganotin(IV) esters having QS value>3 mms− 1 have a five coordinate chain structure formed by bridging carboxylate groups (, ) [Citation30]. On the other hand, 6 displayed QS values 3.04 mms− 1, which recommended trigonal bipyramidal geometry () [Citation31]. On the basis of FT IR and Mössbauer spectra, it was concluded that 1–5 were bridged polymers, while 6 displayed cis-trigonal bipyramidal geometry in the solid phase.
Table II. FT IR data for complexes 1–6 and ligand 7 (cm−1).
Figure 1 (a) Polymeric geometry, (b) tetrahedral geometry, (c) trigonal bipyrmidal geometry and numbering scheme for NMR.
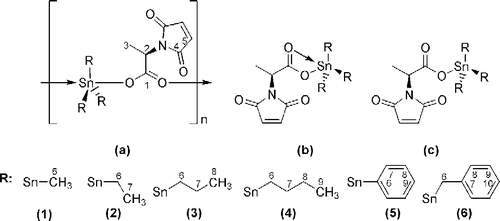
Table III. 119mSn Mössbauer spectral data for complexes 1–6 (mm s−1).
Solution state 1H- 13C- and 119Sn-NMR spectroscopic results
The 1H NMR parameters of the organotin(IV) derivatives of 2-maleimidopropanoic acid are shown in . The integrated intensities of the spectra clearly indicated a 1:1 metal-to-ligand stoichiometry in solution, in agreement with the analytical data on the solids. Tin satellites were observed from which the coupling constants to tin can be obtained. Lockhart's equations (Equations (1) and (2)) using 1J[119Sn-13C] and 2J[119Sn-1H] values respectively were successfully applied to 1–4 for the determination of the C–Sn–C angle, θ [Citation32] (Tables and ).
C–Sn–C derived from Equations (1) and (2), for 1–4 had values of 110°, 108°, 112°, 111° and 111°, 108°, 111°, 108° respectively which confirmed that the hyper-coordination to Sn(IV) was lost in solution [Citation33]. Moreover, 1J[119Sn-13C] provided a trend 1J>>2J < 3J, which also indicated a tetrahedral environment of Sn(IV) [Citation34]. These results clearly recommended a tetrahedral geometry for 1–4 [Citation35]. 119Sn NMR chemical shifts of 1–4 were typical of four-coordinated Sn(IV) complex; while for 5 and 6 peaks at 94.63 and − 104.55 ppm indicated penta-coordination of Sn(IV). These results clearly indicated a tetrahedron for 1–4 and trigonal bipyramid for 5 and 6 in solution [Citation21].
Table IV. 1H NMR data of 1–7 with 2J(119Sn-1H) in parenthesis.
Table V. 13C NMR data of 1–7 with 1J(119Sn-13C) in parenthesis.
MS Spectrometry
FAB-MS substantiated the 1:1 metal-to-ligand stoichiometry and structural hypothesis based upon the aforementioned spectral analyses. The title complexes followed a fragmentation pattern as earlier reported [Citation19,Citation23]. The base peak for 1, 4 and 5 was due to the [LSnR2]+ fragment at m/z 317, 401, 441, respectively; while in 2, 3 and 6 the [LBSnR]+ fragment was at m/z 318, 330 and 378 [Citation36].
Bioactivity
Compounds 1–7 were tested in vitro for their bioavailability, against seven human tumoural cell lines, five human pathogenic fungi and the urease enzyme. lists the concentration that inhibits 50% of the cell growth (ID50) for 2-maleimidopropanoic acid (7) and its tin(IV) complexes (1–7) against A498 renal cancer, EVSA-T mammary cancer, H226 lung cancer, IGROV ovarian cancer, M19 melanoma, MCF-7 mammary cancer and WiDr colon cancer of human tumour origin along with the corresponding ID50 values for the clinically used drugs doxorubicin, cisplatin, 5-fluorouracil, methotrexate and etopside for comparison. All the complexes displayed significant activities in comparison to the ligand and the reference drugs, however, 5 and 6 were found to be the most potent.
Table VI. In vitro inhibition doses ID50 (ng/mL) of compounds 1–7 against seven tumoral cell lines of human origin.
represents concentrations of compounds 1–6 inducing 50% (IC50) and 100% (IC100) mortality for five leishmanial strains. It is quite evident from the data obtained that the complexes displayed significant activities in comparison to the ligand.
Table VII. In vitro anti-leishmanial effect of 1–7 (mM) in promastigote stage.
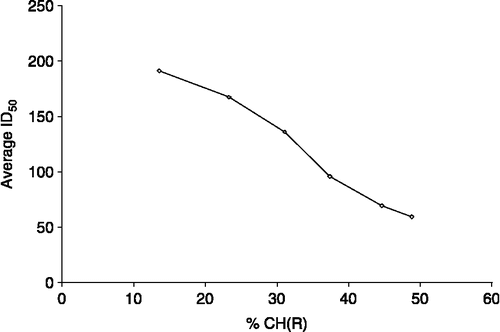
Promising results were observed during in vitro anti-fungal screening. The complexes 1–6 were more effective than the ligand 7. The order of in vitro anti-fungal effectiveness is 6>5>4>3>2>1>>7. The effect of dose over percent inhibition was plotted and from that graph we have determined the Optimum Dose by extrapolating the value up to the extent that a further increase in dose does not effect the inhibition (). The results show a similar trend as their toxicity leading to the conclusion concludes that the higher the toxicity of a compound the lower is the optimum dose.
Table VIII. In vitro anti-fungal effect of 1–7.
shows the inhibitory of 1–7 on urease. It was observed that compounds 5 and 6 displayed a potent inhibitory effect, 3 and 4 were moderate inhibitors while 1, 2 and 7 were weak inhibitors of urease.
Table IX. In vitro inhibition of urease by compounds 1–7.
The bulkiness of R groups attached to Sn(IV), affected in vitro toxicity against the tumoural cell lines used. To highlight this statement, the average ID50 data have been plotted versus the percent CH in . The percent CH has been defined as:
Where n is the number of carbon or hydrogen atoms in the R group.
In , ID50 decreases almost linearly with the increase in percent CH. However, some deviations in the case of alkyl R groups have been observed, which may be attributed to variation in conformational behavior and distribution of complexes between phases.
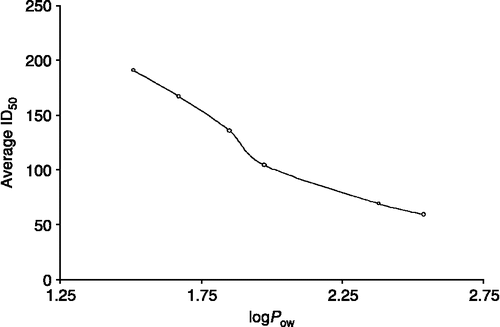
Since it is difficult to judge the bioactivity of any compound by a single factor, we have tried another important parameter, the partition coefficient and the ID50 values have been plotted versus ID50 (see ). It is interesting to note that the data for complexes 1–6 show that the ID50 values decrease linearly with the increase in partition coefficient. It is certainly encouraging to us that the major controlling parameter seems to be Pow (partition coefficient in octanol/water system) or, in other words, the polarizibility of the R′–Sn bond induced by R groups. It can be therefore be concluded that in complexes 1–6, lipophillicity increases with the bulkiness of R groups, which enhances the partition coefficient, thereby increasing the bioactivity. Moreover, the polarity of Sn–C is also of the same order (Bz>Ph>Bu>Pr>Et>Me). In conclusion, we can say that the bulkiness of the attached R group/percent CH values and polar character of carboxylic group of 2-maleimidopropanoic acid are interlinked with each other, which enhance the polarity and partition coefficients of the complexes. A study is currently being carried out for the in vivo interactions/mechanism of action of these complexes.
Acknowledgements
This work was carried out with the financial support of Gomal University, D. I. Khan, Pakistan, (Research Project No. 717-29/DF/GU).
References
- Maqsood ZT, Khalid KM, Ashiq U, Jamal RA, Chohan ZH, Mahroof-Tahir M, Supuran CT. Oxovanadium(IV) complexes of hydrazides: Potential antifungal agents. J Enzyme Inhib Med Chem 2006; 21: 37, DOI:10.1080/14756360500277459j.jorganchem.2005.10.007T
- Chohan ZH, Supuran CT. In-vitro antibacterial and cytotoxic activity of cobalt (II), copper (II), nickel (II) and zinc (II) complexes of the antibiotic drug cephalothin (Keflin). J Enzyme Inhib Med Chem 2005; 20: 463, DOI:10.1080/104852505002213280
- Rehman SU, Chohan ZH, Gulnaz F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J Enzyme Inhib Med Chem 2005; 20: 333, DOI:10.1080/14756360500141911
- Lickiss PD. Chemistry of tin, PJ Smith. Blackie, London 1998
- Van Laar JAM, Rustam YM, Van der Vit C, Smid K, Kuiper CM, Pinedo HM, Peters GJ. Tumor size and origin determine the antitumor activity of cisplatin or 5-fluorouracil and its modulation by leucovorin in murine colon carcinomas. Cancer Chemother Pharmacol 1996; 39: 79
- Girasolo MA, Schillaci D, Di Salvo C, Barone G, Silvestri A, Ruisi G. Synthesis, spectroscopic characterization and in vitro antimicrobial activity of diorganotin(IV) dichloride adducts with [1,2,4]triazolo-[1,5-a]pyrimidine and 5,7-dimethyl-[1,2,4]triazolo-[1,5-a]pyrimidine. J Organomet Chem 2005; 961: 963, DOI:10.1016/j.jorganchem.2005.10.007
- Ronconi L, Marzano C, Russo U, Sitran S, Graziani R, Fregona D. Synthesis, characterization and in vitro cytotoxicity of new organotin(IV) derivatives of N-methylglycine. J Inorg Biochem 2002; 91: 413, DOI:10.1016/S0162-0134(02)00465-8
- Marchetti F, Pellei M, Pettinari C, Pettinari R, Rivarola E, Santini C, Skelton BW, White AH. Tin(IV) and organotin(IV) derivatives of bis(pyrazolyl)acetate: Synthesis, spectroscopic characterization and behaviour in solution.: X-ray single crystal study of bis(pyrazol-1-yl)acetatotri-iodotin(IV) [SnI3(bdmpza)]. J Organomet Chem 2005; 690: 1878, DOI:10.1016/j.jorganchem.2005.01.067
- Thoonen SHL, Deelman B-J, van Koten G. Synthetic aspects of tetraorganotins and organotin(IV) halides. J Organomet Chem 2004; 689: 2145, DOI:10.1016/j.jorganchem.2004.03.027
- Pellerito L, Nagy L. Organotin(IV)n+ complexes formed with biologically active ligands: Equilibrium and structural studies, and some biological aspects. Coord Chem Rev 2002; 224: 111, DOI:10.1016/S0010-8545(01)00399-X
- Meriem A, Gielen M, Willem R. Synthesis, characterization and antitumour activity of a series of diorganotin(IV) derivatives of bis(carboxymethyl)amines. J Organomet Chem 1989; 365: 91, DOI:10.1016/0022-328X(89)87170-0
- Yin HD, Wang QB, Xue SC. Synthesis and spectroscopic properties of [N-(4-carboxyphenyl) salicylideneiminato] di- and tri-organotin (IV) complexes and crystal structures of [nBu2Sn(2-OHC6H4CH = NC6H4COO)]2O2 and Ph3Sn(2-OHC6H4CH = NC6H4COO). J Organomet Chem 2005; 690: 435, DOI:10.1016/jjorganchem200409063
- Zhou Y, Jiang T, Ren S, Yu J, Xia Z. Synthesis, crystal structure and in vitro antitumor activity of di-n-butyltin 4′-(7-oxabicyclo [2,2,1]-5-heptane-2,3-dicarboximide) benzoates. J Organomet Chem 2005; 690: 2186, DOI:10.1016/j.jorganchem.2005.01.062
- Harrison PG, Sharpe NW. Complexes of terminally-protected dipeptides with trimethyltin as models for triorganotin-protein interactions. Inorg Chim Acta 1985; 108: 7, DOI:10.1016/S0020-1693(00)84315-7
- Sandhu GK, Gupta R, Sandhu SS, Parish RV, Brown K. Diorganotin(IV) derivatives of N-phthaloyl amino acids. J Organomet Chem 1985; 279: 373, DOI:101016/0022-328X(85)87035-2
- Sandhu GK, Gupta R, Sandhu SS, Parish RV. Diorganotin(VI) complexes of N-acetylamino acids. Polyhedron 1985; 4: 81, DOI:10.1016/S0277-5387(00)84226-6
- Sandhu GL, Hundal R, Tiekink ERT. Structural chemistry of organotin carboxylates : XVII. Diorganotin(IV) derivatives of N-phthaloyl-DL-valine. J Organomet Chem 1992; 430: 15, DOI:10.1016/0022-328X(92)80091-B
- Lo K-M, Kumar Das VG, Yip W-H, Mak TCW. Organotin esters of 3-ureidopropionic acid. Crystal structure of triphenyltin(IV) 3-ureidopropionate, (C6H5)3SnOCO(CH2)2NHCONH2. J Organomet Chem 1991; 412: 21, DOI:10.1016/0022-328X(91)86037-Q
- Khan MI, Baloch MK, Ashfaq M, Peters GJ. In vivo toxicological effects and spectral studies of Organotin(IV) N-maleoylglycinates. Appl Organomet Chem 2005; 19: 132, DOI:10.1002/aoc807
- Khan MI, Baloch MK, Ashfaq M. Biological aspects of new Organotin(IV) compounds of 3-maleimidopropionic acid. J Organomet Chem 2004; 689: 3370, DOI:10.1016/j.jorgan.chem.2003.10.007
- Ashfaq M, Khan MI, Baloch MK, Malik A. Biologically potent Organotin(IV) complexes of 2-maleimidoacetic acid. J Organomet Chem 2004; 689: 238, DOI:10.1016/j.jorganchem.2003.10.007
- Khan MI, Baloch MK, Ashfaq M, Obaidullah. Synthesis, characterization and in vitro cytotoxic effects of new organotin(IV)-2-maleimidopropanoates. Appl Organomet Chem 2006; 20: 463, DOI:10.1002/aoc1083
- Khan MI, Baloch MK, Ashfaq M, Stoter G. In vivo toxicological effects and spectral studies of new triorganotin(IV)-N-maleoyltranexamates. J Organomet Chem 2006; 691: 2554, DOI:10.1016/j.jorganchem.2006.01.057
- Sisido K, Takeda Y, Kinugawa Z. Direct Synthesis of Organotin Compounds. I. Di- and Tribenzyltin Chlorides. J Am Chem Soc 1961; 83: 538
- Furniss B, Hannaford AJ, Smith PWG, Tatchell AR. Vogel's text book of practical organic chemistry. ELBS Longman Group, UK 1989
- Mendham J, Denney RC, Barnes JD, Thomas M. Vogel's text book of quantitative chemical analysis. Pearson Education Pte. Ltd, Singapore 2003
- Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 1967; 39: 971, DOI: 10.1021/ac60252a045
- Lebl T, Holecek J, Lycka A. Sci Pap Univ Pardubice Ser 1996; 5: A2
- Whiffen DH. Vibrational frequencies and thermodynamic properties of fluoro-, chloro-, bromo and iodo-benzene. J Chem Soc 1956; 1350, DOI:10.1039/JR9560001350
- Smith PJ, Day RO, Chandrasekhar V, Holmes JM, Holmes RR. Pentacoordinated molecules. 66. Chain structures of trimethyltin esters of salicylic acid and o-anisic acid. Tin-119m Moessbauer study of a series of trimethyltin and triphenyltin carboxylates. Inorg Chem 1984; 25: 2495, DOI: 10.1021/ic00235a005
- Sharma HK, Lata S, Sharma KK. Molloy KC,Waterfield PC. Synthesis and spectroscopic studies on dibutyl-, tributyl- and triphenyltin esters of p-methoxy trans-cinnamic acid. J Organomet Chem 1988; 315: 9, DOI: 10.1016/0022-328X(88)80294-8
- Lockhart TP, Manders WF, Holt EM. Metal-stabilized rare tautomers of nucleobases. 1. Iminooxo form of cytosine: Formation through metal migration and estimation of the geometry of the free tautomer. J Am Chem Soc 1986; 108: 6611, DOI: 10.1021/ja00281a027
- Willem R, Bouhdid A, Mahieu B, Ghys L, Biesemans M, Tiekink ERT, Vos D de, Gielen M. Synthesis, characterization and in vitro antitumour activity of triphenyl- and tri-n-butyltin benzoates, phenylacetates and cinnamates. J Organomet Chem 1997; 531–151, DOI: 10.1016/S0022-328X(96)06686-7
- Dalil H. A novel approach to soluble polystyrenes functionalized by tri-n-butyltin carboxylates. Macromol Chem Phys 2000; 201: 1266, DOI: 10.1002/1521-3925(20000801)201:12 < 1266::AID-MACP1266>3.0.CO;2-O
- Pellerito A, Fiore T, Giuliani AM, Maggio F, Pellerito L, Vitturi R, Colomba MS, Barbieri R. Organometallic Complexes with Biological Molecules: VIII. Synthesis, Solid State and in vivo Investigation of Triorganotin(IV) Derivatives of L-Homocysteic Acid. Appl Organomet Chem 1997; 11: 601, DOI: 10.1002/(SICI)1099-0739(199707)11:7>601::AID-AOC616>3.0.CO;2-1
- López-Torres E, Mendiola MA, Pastor CJ, Procopio JR. Diorganotin(iv) Complexes of a Cyclic Thiosemicarbazone Ligand: Crystal Structures of [SnPh2(C15H10N3S)Cl] and [SnMe2(C15H10N3S)2]. Eur J Inorg Chem 2003; 2711, DOI: 10.1002/ejic.200300090