Abstract
Glycyrrhizin and its aglycone, glycyrrhetic acid has been found useful for various therapeutic purposes. Glycyrrhizin has been shown to possess many physiological functions like anti-inflammatory activity, detoxification and inhibition of carcinogenic promoters. 12-O-Tetradecanoyl phorbol-13-acetate (TPA), a well-known phorbal ester is known for its tumor promotion activity. The induction of inflammation in skin mediated by TPA is believed to be governed by cyclooxygenase (COX), lipoxygenase and ornithine decarboxylase (ODC). These markers of inflammatory responses are important for skin tumor promotion. In our present study, we studied the chemopreventive effect of glycyrrhizin on TPA (20 nmol/0.2 mL acetone/animal, topically)-induced oxidative stress and hyperproliferation markers in skin. TPA enhanced lipid peroxidation with reduction in the level of catalase, glutathione, glutathione peroxidase, glutathione reductase and glutathione-s-transferase. TPA treatment also enhanced ODC activity and [3H] thymidine incorporation into cutaneous DNA. Prophylactic treatment of mice with glycyrrhizin (2.0 & 4.0 mg/0.2 mL acetone/animal, topically) resulted in a significant decrease in cutaneous microsomal lipid peroxidation (P < 0.001) and recovery of cutaneous glutathione content (P < 0.001) and its dependent enzymes. A significant inhibition in ODC activity and DNA synthesis (P < 0.001) was also observed. Thus, the results demonstrate that pretreatment with glycyrrhizin is protective against TPA-induced oxidative stress and tumor promotion in Swiss albino mice.
Introduction
In developing countries the treatment of diseases with medicinal plants and dietary products are still popular [Citation1] and several investigations have been conducted on the chemical constituents and biological activities of medicinal plants. The induction of oxidative stress, ornithine decarboxylase (ODC) activity and DNA synthesis occurs during tumor promotion with tumor promoters such as lauryl peroxide, benzoyl peroxide and 12-O-tetradecanoyl phorbol-13-acetate (TPA) [Citation2]. The mouse skin tumor promoter TPA has been reported to increase release of reactive oxygen species (ROS) like H2O2 production in murine epidermal keratinocytes and induces oxidative DNA damage. Oxidative stress results from the disturbance in the equilibrium status of pro-oxidant/antioxidant systems such as when an increase in oxidant generation takes place at the same time as a decrease in antioxidant protection occurs which causes oxidative damage to lipids, proteins, carbohydrates and nucleic acids, ultimately leading to cell death in severe oxidative stress. Oxidative stress is one of the main causes of cytotoxicity or genotoxicity. Thus, oxidative stress is considered to be associated with many diseases, e.g. aging, cardiovascular diseases and cancer [Citation3]. Cells have multiple protective mechanisms against oxidative stress and succeed in preventing cell damage. Many dietary constituents are important sources of protective agents that range from antioxidants, vitamins and minerals to food additives that might enhance the action of natural antioxidants [Citation4].
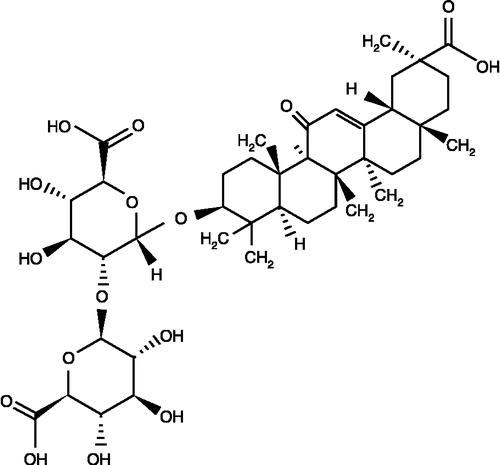
Glycyrrhizin (), a conjugate of one molecule of glycyrrhetinic acid and two molecules of glucuronic acid, is the main constituent of Glycyrrhiza glabra L (licorice) [Citation5]. It is a sweet substance, which is 150–300 times sweeter than sugar. It has many physiological functions such as anti-inflammatory activity, detoxification and inhibition of carcinogenic promoters. Glycyrrhizin is considered to be the most common of the Asiatic folk medicines that acts as an anti-inflammatory agent on neutrophil functions including ROS generation [Citation6]. Thus, glycyrrhizin can be considered as a quenching agent of free radicals and a blocking agent of lipid peroxidation chain reactions.
Therefore, in the present study we have tested the efficacy of glycyrrhizin against TPA-induced early tumor promotion markers such as oxidative stress and hyperproliferation in mouse skin.
Materials and methods
Chemicals
Reduced glutathione (GSH), oxidized glutathione (GSSG), nicotinamide adeninedinucleotide phosphate reduced (NADPH), thiobarbituric acid (TBA), trichloroacetic acid (TCA), bovine serum albumin (BSA), 1,2,dithio-bis-nitrobenzoic acid (DTNB), 1-chloro-2,4,dinitrobenzene (CDNB), glutathione reductase, 12-O-tetradecanoyl phorbol-13-acetate (TPA) and glycyrrhizin were obtained from Sigma Chemicals Co (St.Louis, MO). Ascorbic acid, hydrogen peroxide, ferric chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate and sodium hydroxide were purchased from E. Merck, India. All other chemicals were of the highest purity commercially available.
Animals
Eight-week-old adult female Swiss albino mice (20–25 g) were obtained from the Central Animal House Facility of Hamdard University, New Delhi and were housed in a ventilated room at 25°C under a 12-h light/dark cycle. The mice were allowed to acclimatize for one week before the study and had free access to standard laboratory feed (Hindustan Lever Ltd, Bombay, India) and water ad libitum. The dorsal skin of the mice was shaved with an electric clipper (Oster A2) followed by the application of hair removing cream (Anne French, Geoffrey Manners, Bombay, India) at least 2 days before treatment. Only mice showing no signs of hair regrowth were used for experiments.
Experimental protocol
To study the effect of pretreatment with glycyrrhizin on TPA-mediated cutaneous oxidative stress, thirty female Swiss albino mice were randomly allocated to 5 groups of 6 mice each. Group I received only acetone (0.2 mL/animal). Group II received only TPA (20 nmol/0.2 mL acetone/animal). Group III received pretreatment glycyrrhizin (2.0 mg/0.2 mL acetone/animal) + TPA (20 nmol/0.2 mL acetone/animal). Group IV received glycyrrhizin (4.0 mg/0.2 mL acetone/animal) + TPA (20 nmol/0.2 mL acetone/animal). Group V received glycyrrhizin (4.0 mg/0.2 mL acetone/animal) only. After 12 h, the animals were sacrificed by cervical dislocation and processed for sub-cellular fractionation.
To study the effect of pre-treatment of animals with glycyrrhizin on TPA-mediated induction of cutaneous ODC activity, 30 female mice were randomly allocated to five groups of six mice in each. The animals of group I received topical application of acetone (0.2 mL per animal) and served as a control. The animals of groups III, IV received a single topical application of glycyrrhizin at the dose level of 2.0 and 4.0 mg kg− 1 body weight, respectively, in acetone. One hour later after the treatment of glycyrrhizin, the animals of groups II, III, IV received a single topical application of TPA (20 nmol per animal per 0.2 mL acetone). While group V received only glycyrrhizin at the level of 4 mg kg− 1, in acetone. All these mice were killed 6 h after the treatment by cervical dislocation. The skin was quickly removed and processed for sub-cellular fractionation.
For studying the effect of glycyrrhizin on TPA-mediated [3H] thymidine incorporation into cutaneous DNA, the experimental protocol was exactly similar to that described for oxidative stress. One hour after the last treatment of the animals with glycyrrhizin, the animals of groups III, IV and V received topical application of TPA. Eighteen hours after treatment with TPA, the animals of all the groups were given [3H] thymidine (15 μci/animal/0.2 mL saline) as an i.p. injection and were sacrificed after 2 h by cervical dislocation. Their skin tissues were quickly removed cleaned free of extraneous material and homogenized in cold distilled water for further processing and separation of DNA.
Tissue preparation
After the desired time period, control and treated animals were sacrificed by cervical dislocation. The animals were immediately dissected to remove their skin, which was washed in ice cold saline (0.85 M NaCl) and extraneous materials were removed. All subsequent operations were carried out on ice at a temperature not above 4°C.
Preparation of postmitochondrial supernatant (PMS) and microsomes
For biochemical studies, a known amount of tissue was minced and homogenized in chilled phosphate buffer (0.1 M, pH 7.4) containing KCl (1.17%) using a polytron homogenizer (Kinematica AGPT 3000). The homogenate was filtered through a muslin cloth and was centrifuged at 88 × g for 15 min at 4°C in an Elton refrigerated centrifuge (RC 4100D) to separate the nuclear debris. The supernatant obtained was used as a source of enzymes. A portion of the post mitochondrial supernatant (PMS) was centrifuged in an ultracentrifuge (Beckman, L7-55) at 100500 × g (34000 rpm) for 60 min at 4°C. The pellet was considered to be the microsomal fraction and was suspended in phosphate buffer (0.1 M, pH 7.4) containing KCl (1.17%). The supernatant (cytosolic fraction) was used as a source of phase II detoxifying enzymes such as glutathione S-transferase.
Biochemical estimations
Estimation of lipid peroxidation
The assay of lipid peroxidation was done according to the method of Wright et al. [Citation7]. The reaction mixture consisted of 0.58 mL phosphate buffer (0.1 M, pH 7.4), 0.2 mL microsomes, 0.2 mL ascorbic acid (100 mM) and 0.02 mL ferric chloride (100 mM) in a total of 1 mL. This reaction mixture was then incubated at 37°C in a shaking water bath for 1 h. The reaction was stopped by the addition of 1 mL of TCA (10%). Following addition of 1.0 mL TBA (0.67%), all the tubes were placed in a boiling water bath for a period of 20 min. The tubes were then transferred to an ice bath and centrifuged at 2500 × g for 10 min. The amount of malondialdehyde (MDA) formed in each of the samples was assessed by measuring the optical density of the supernatant at 535 nm. The results were expressed as nmol MDA formed/h/g tissue at 37°C by using a molar extinction coefficient of 1.56 × 105 M− 1 cm− 1.
Estimation of glutathione reduced
The reduced glutathione (GSH) in skin was determined by the method of Jollow et al. [Citation8]. A 1.0 mL postmitochondrial supernatant fraction (PMS) (10%) was mixed with 1.0 mL sulphosalicylic acid (4%) and the samples incubated at 4°C for at least 1 h and then centrifuged at 1200 × g for 15 min at 4°C. The reaction mixture contained 0.4 mL of the filtered sample, 2.2 mL phosphate buffer (0.1 M, pH 7.4) and 0.4 mL DTNB (4 mg/mL) in a total volume of 3.0 mL. The yellow color developed was read immediately at 412 nm on a spectrophotometer (Milton Roy Model-21 D) and the reduced glutathione concentration was calculated as nmol GSH/g tissue.
Assay for glutathione S–transferase activity
Glutathione S-transferase (GST) activity was assayed by the method of Habig et al. [Citation9]. The reaction mixture consisted of 2.5 mL phosphate buffer (0.1 M, pH 6.5), 0.2 mL GSH (1 mM), 0.2 mL CDNB (1 mM) and 0.1 mL of the cytosolic fraction (10%) in a total volume of 3.0 mL. Changes in absorbance were recorded at 340 nm and enzymatic activity was calculated as nmol CDNB conjugate formed/min/mg protein using a molar extinction coefficient of 9.6 × 103 M− 1 cm− 1.
Assay for glutathione reductase activity
Glutathione reductase activity was assayed by the method of Carlberg and Mannervick [Citation10]. The reaction mixture consisted of 1.65 mL phosphate buffer (0.1 M, pH7.6), 0.1 mL EDTA (0.5 mM), 0.05 mL oxidized glutathione (1 mM), 0.1 mL NADPH (0.1 mM) and 0.1 mL PMS (10%) in a total volume of 2.0 mL. Enzyme activity was determined at 25°C by measuring the disappearance of NADPH at 340 nm and was calculated as nmol NADPH oxidized/min/mg protein using a molar extinction coefficient of 6.22 × 103 M− 1 cm− 1.
Assay for catalase activity
Catalase activity was assayed by the method of Claiborne [Citation11]. Briefly, the reaction mixture consisted of 2.0 mL phosphate buffer (0.1 M, pH 7.4), 0.95 mL hydrogen peroxide (0.019 mM) and 0.05 mL PMS (10%) in a final volume of 3.0 mL. Changes in absorbance were recorded at 240 nm and catalase activity was calculated as nmol H2O2 consumed/min/mg protein.
Assay for glutathione peroxidase activity
Glutathione peroxidase activity was measured by the method of Mohandas et al. [Citation12]. The reaction mixture consisted of 1.44 mL phosphate buffer (0.1 M, pH7.4), 0.1 mL EDTA (1 mM), 0.1 mL sodium azide (1 mM), 0.05 mL glutathione reductase (1 IU/ml), 0.05 mL reduced glutathione (1 mM), 0.1 mL NADPH (0.2 mM) and 0.01 mL H2O2 (0.25 mM) and 0.1 mL 10% PMS in a total volume of 2 mL. The disappearance of NADPH at 340 nm was recorded at 25°C and enzyme activity was calculated as nmol NADPH oxidized/min/mg protein using a molar extinction coefficient of 6.22 × 103 M− 1 cm− 1.
Assay for ornithine decarboxylase activity
ODC activity was determined using 0.4 mL cutaneous 105,000 × g supernatant fraction per assay tube by measuring the release of CO2 from DL- [14C] ornithine by the method of O'Brien et al. [Citation13]. The skin was homogenized in Tris-HCI buffer (pH 7.5, 50 mM) containing EDTA (0.4 mM), pyridoxal phosphate (0.32 mM), PMSF (0.1 mM), 2-mercaptoethanol (1.0 mM), dithiothreitol (4.0 mM) and Tween 80 (0.1%) at 4°C using a polytron homogenizer (Kinematica AGPT 3000). In brief, the reaction mixture contained 400 μL enzymes and 0.095 mL co-factor mixture containing pyridoxal phosphate (0.32 mM), EDTA (0.4 mM), dithiothreitol (4.0 mM), ornithine (0.4 mM), Brij 35 (0.02%) and DL-[14C] ornithine (0.05μCi) in a total volume of 0.495 mL. After adding buffer and co-factor mixture to the blank and others tubes, the tubes were closed immediately with a rubber stopper containing 0.2 mL ethanolamine and methoxyethanol mixture (2:1) in the central well and kept in a water-bath at 37°C. After 1 hr of incubation, the enzyme activity was arrested by injecting 1.0 mL citric acid solution (2.0 M) along the sides of the glass tubes and the solution was continued for 1 h to ensure complete absorption of CO2. Finally, the central well was transferred to a vial containing 2 mL ethanol and 10 mL toluene-based scintillation fluid. Radioactivity was counted in a liquid scintillation counter (LKB Wallace-1410). ODC activity was expressed as pmol CO2 released/h/mg protein.
Quantitation of hepatic DNA synthesis
The isolation of skin DNA and assessment of incorporation of [3H] thymidine into DNA was done by the method employed by Smart et al. [Citation14]. The skin tissues were quickly excised from the animal, cleaned free of extraneous material and a homogenate (20%) was prepared in ice-cold water. The precipitate thus obtained was washed with cold trichloroacetic acid (5%) and incubated with cold perchloric acid (10%) at 4°C for 24 h. The mixture was then centrifuged and the precipitate was washed with cold perchloric acid (5%). Then the precipitate was dissolved in warm perchloric acid (5%) followed by incubation in a boiling water bath for 30 min and filtered through a Whatman 50 paper. The filtrate was used for [3H]thymidine counting in a liquid scintillation counter (LKB-Wallace-1410) after adding scintillation fluid. The amount of DNA in the filtrate was estimated by the diphenylamine method of Giles and Mayers (1965). The amount of [3H] thymidine incorporated was expressed as dpm/μg DNA.
Protein estimation
The protein concentration in all samples was determined by the method of Lowry et al. [Citation15] using bovine serum albumin (BSA) as standard.
Statistical analysis
The level of significance between different groups is based on analysis of variance test followed by Dunnett's t test. The P values of less than 0.05 have been considered significant.
Results
shows the effect of glycyrrhizin on levels of TPA-mediated cutaneous glutathione, and glutathione-S-transferase in murine skin. TPA caused 31% decrease in GSH and 18% decrease in GST when compared with the control group. Treatment with glycyrrhizin (at 2.0 mg and 4.0 mg/0.2 mL acetone/animal) caused 5–13% and 1–14% elevation in the levels of GSH and GST respectively when compared with the TPA group.
Table I. Effect of prophylactic treatment of animals with glycyrrhizin on TPA–mediated cutaneous glutathione, and glutathione-S-transferase in murine skin.
shows the effect of glycyrrhizin on levels on TPA-mediated cutaneous glutathione peroxidase (Gpx) and glutathione reductase (GR) in murine skin. TPA caused 22% and 55% reduction in Gpx and GR respectively when compared with the acetone group. Treatment with glycyrrhizin (at 2.0 mg and 4.0 mg/0.2 mL acetone/animal) caused 5–14% and 15–34% elevation in levels of GPx and GR, respectively, when compared with the TPA group.
Table II. Effect of prophylactic treatment of animals with glycyrrhizin on TPA-mediated cutaneous glutathione peroxidase and glutathione reductase in murine skin.
shows the effect of glycyrrhizin on levels on TPA mediated cutaneous catalase and LPO in murine skin. TPA caused 70% reduction in catalase and 32% elevation in LPO when compared with acetone group. Treatment with glycyrrhizin (at 2.0 mg and 4.0 mg/0.2 mL acetone/animal) caused 19–32% elevation in levels of catalase and 59–67% decrease in LPO when compared with the TPA group.
Table III. Effect of prophylactic treatment of animals with glycyrrhizin on TPA-mediated cutaneous catalase and lipid peroxidation in murine skin.
The effect of pre-treatment of animals with glycyrrhizin on TPA-mediated induction of cutaneous ODC activity is shown in . TPA treatment resulted in 364% induction in the ODC activity as compared with the acetone-treated controls. The pretreatment of mice with glycyrrhizin at a dose of 2.0 mg/0.2 mL acetone/animal caused 69% and at a dose of 4.0 mg/0.2 mL acetone/animal caused 178% inhibition in the elevation of ODC activity as compared with the TPA treated control group.
Figure 2 Effect of pretreatment of mice with Glycyrrhizin on TPA – mediated enhancement of ornithine decarboxylase (ODC) activity and 3H incorporation in skin DNA in mice. Each value represents mean ± SE of six animals. Acetone-treated group served as control. Values marked with asterisks differ significantly from the corresponding value for mice treated with TPA – treated control (***p < 0.001). Values marked with hatch differ significantly from the corresponding value for acetone-treated control (###p < 0.001).
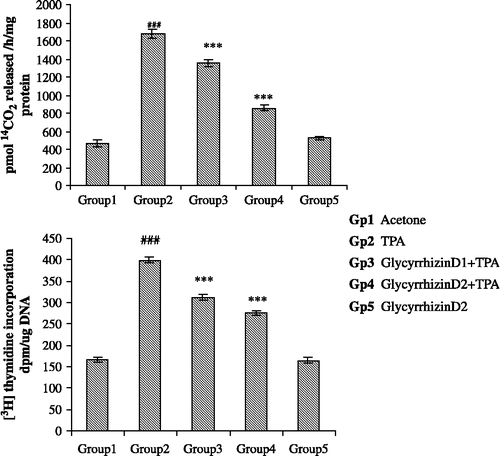
The effect of pre-treatment of animals with glycyrrhizin on TPA-mediated enhancement of the incorporation of [3H] thymidine into skin DNA is shown in . TPA treatment resulted in 239% increase in the incorporation of [3H] thymidine into skin DNA as compared with the acetone-treated controls. Treatment with glycyrrhizin at a dose of 2.0 mg/0.2 mL acetone/animal caused 52% and at a dose of 4.0 mg/0.2 mL acetone/animal caused 74% inhibition in the incorporation of [3H] thymidine skin DNA when compared with the TPA-treated control group.
Discussion
Oxidative stress occurs when the generation of reactive oxygen species (ROS) in a system exceeds the system's ability to neutralize and eliminate them [Citation16]. The imbalance can result from a lack of antioxidant capacity caused by disturbance in production, distribution or by overabundance of ROS from an environment or behavioral stress. If not regulated properly from generation it leads to the formation of highly reactive species, hydroxyl radicals, that attack proteins and lipid membranes causing cell damage or genetic alterations, inhibiting normal functions [Citation17]. It has been found that oxidative stress also occurs during tumor promotion as a result of which oxidative stress has been implicated in a growing list of human diseases as well as in the ageing process [Citation18].
TPA has been shown to act as a strong tumor promoter [Citation19]. Treatment with TPA has been reported to induce a variety of changes in murine skin, including dark basal keratinocytes and sustained epidermal hyperplasic, reactive oxygen formation in epidermis, elevated epidermal cyclooxygenase, lipoxygenase activities, DNA synthesis and epidermal ODC activity leading to an increase in polyamine biosynthesis [Citation20]. Diets containing an abundance of fruits & vegetables are protective against a variety of diseases, particularly cardiovascular disease and cancer [Citation21, Citation22]. Triterpenoids, a major group of phytochemicals, have also been reported to have anticarcinogenic effects [Citation23] although the mechanisms by which they display anti-carcinogenic effects are not clear.
On the basis of the pharmacological effects of glycyrrhizin, including its anti-inflammation and detoxifying property we have selected this compound for the present study. Glycyrrhizin is a triterpenoid saponin which exerts an inhibitory effect on neutrophil migration to the sites of inflammation [Citation24]. Inflammatory cells produce biologically active arachidonic acid metabolites including lipid hydroperoxides, prostanoids and leukotrienes and additionally posses the capacity to generate and release reactive oxygen species (ROS) and free radicals during an oxidative state Citation25-27. Skin epidermis is a tissue showing arachdonic acid metabolites including lipid peroxidation; prostaglandins and leukotrienes have been widely accepted as being involved in inflammatory process Citation28-30.
From our results, we found that the pretreatment of animals with glycyrrhizin significantly ameliorated the GSH content with a concomitant decrease in lipid peroxidation. It could be suggested that glycyrrhizin has established antioxidant properties that might have counteracted the toxic effects of TPA by effectively scavenging and blocking dose dependently the conjugation of reactive intermediates to GSH as evident from the partially recovered GSH content and decreased MDA formation. TPA administration resulted in a concomitant decrease in GPx, GR, and GST activities. Pretreatment with glycyrrhizin and subsequent exposure of mice with TPA led to significant increase in the above antioxidant enzymes. This might have been possible because GST, GR and GPx are secondary oxidant enzymes which might have helped in the detoxification of reactive oxygen species by decreasing the peroxide level or by maintaining a steady supply of glutathione [Citation31]. Considering the importance of oxidative damage in carcinogenesis, induction of the detoxification of enzymes by naturally occurring or synthetic agents represents a promising strategy for cancer prevention [Citation32]. Besides, it has been shown in one of the studies that the balance between phase I carcinogen activating enzymes and phase II detoxifying enzymes is important in determining the risk of developing chemically induced cancer subsequent to tea consumption [Citation33]. It has been postulated that glycyrrhizin possesses anti-carcinogenic properties and they induce a variety of enzymes involved in the detoxification and excretion of carcinogenic and toxic substances.
ODC activity and [3H] thymidine incorporation are widely used as biochemical markers to evaluate the tumour-promoting potential of an agent. Elevation in ODC activity and [3H] thymidine incorporation is involved in the proliferative and tumor promoting activity of TPA. Several inhibitors of ODC and DNA synthesis have been reported to inhibit tumor promotion in various organs [Citation34] and similarly in this study glycyrrhizin dose- dependently inhibited the induction of ODC activity and [3H] thymidine incorporation.
On the basis of our results, we can conclude that glycyrrhizin is an effective chemopreventive agent having strong antioxidant and antiproliferative activity.
| Abbreviations | ||
| TPA: | = | 12-O-tetradecanoyl phorbol-13-acetate |
| GSH: | = | Reduced glutathione content |
| GST: | = | Glutathione-S-transferase |
| ODC: | = | Ornithine decarboxylase |
| CDNB: | = | 1-chloro-2,4-dinitrobenzene |
| NADPH: | = | Reduced nicotinamide adenine dinucleotide phosphate |
| EDTA: | = | Ethylenediamine tetra acetic acid |
| dpm: | = | Disintegrations per minute |
References
- Qureshi S, Shah AH, Tariq M, Ageel AM. Studies on herbal aphrodisiacs used in Arab system of medicine. Amer J Chinese Med 1989; 17: 57–63
- Lee SF, Liang YC, Lin JK. Inhibition of 1,2,4-benzenetriol-generated active oxygen species and induction of phase II enzymes by green tea polyphenols. Chem Biol Interact 1995; 98: 283–301
- Cerutti PA. Oxy-radicals and cancer. The Lancet 1994; 344: 862–863
- Zhao J, Wang J, Chen Y, Agarwal R. Antitumor promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two stage initiation–promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis 1999; 20: 1737–1745
- Stromer FC, Reisted R, Alexander J. Glycyrrhizic acid in liquorice: Evaluation of health hazard. J Food Chem Toxicol 1993; 31: 303–312
- Akamatsu H, komuto J, Asada Y, Niwa Y. Mechanism of anti-inflammatory action of glycyrrhizin: Effect on neutrophil functions including reactive oxygen species generation. Planta Med 1991; 57: 119–121
- Wright JR, Colby HD, Miles PR. Cytosolic factors that affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys 1981; 206: 296–304
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974; 11: 151
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249: 7130–7139
- Carlberg I, Mannervik B. Glutathione reductase level in rat brain. J Biol Chem 1975; 250: 5475–5480
- Claiborne A. Catalase activity. CRC handbook of methods in oxygen radical research, RA Greenwald. CRC Press, Boca Raton 1985; 283–284
- Mohandas M, Marshal JJ, Duggin GG, Horvath JS, Tiller D. Differential distribution of glutathione and glutathione related enzymes in rabbit kidney. Cancer Res 1984; 44: 5086–5091
- O'Brien TG, Simsiman RC, Boutwell RK. Induction of the polyamine-biosynthetic enzymes in mouse epidermis and their specificity for tumor promotion. Cancer Res 1975; 35: 1662–1670
- Smart HC, Huang MT, Conney AG. Sn -1,2-Diacyglycerols mimic the effects of 12-O-tetradecanoylphorbol-13-acetate in vivo by inducing biochemical changes associated with tumor promotion in mouse epidermis. Carcinogenesis 1986; 7: 1865–1870
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–275
- Sun Y. Free radicals, antioxidants enzymes and carcinogenesis. Free Radic Biol Med 1990; 8: 583–599
- Halliwell B. Oxidants and human disease: Some new concepts. FASEB J 1987; 1: 441–445
- Stadtman ER. Protein oxidation and ageing. Science 1992; 257: 1220–1224
- Huachen W, Krystgna F. In vivo formation of oxidized DNA base in tumor promoter-treated mouse skin. Cancer Res 1991; 51: 4443
- Katiyar SK, Agarwal R, Mukhtar H. Inhibition of 12-O-tetradecanoyl phorbal acetate caused tumor promotion in 7,12, dimethyl benz (a) anthracene-initiated Sincar mouse by a polyphenol fraction. Cancer Res 1996; 56: 1023–1030
- Xiaoguang C, Hongyan L, Xiaohong L, Zhaodi F, Yan L, Lihua T, Rui H. Cancer chemopreventive and therapeutic activities of red ginseng. J ethanopharm 1998; 60: 71–78
- Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. Cell Biochem 1995; 22: 169–180
- Tanaka R, Minami T, Ishikawa Y, Matsunaga S, Tokuda H, Nishino H. Cancer chemopreventive activity of serratane-type triterpenoids on two-stage mouse skin carcinogenesis. Cancer Lett 2003; 196: 121–126
- Francischetti IMB, Monteiro RQ, Guimaraes JA. Identification of glycyrrhizin as a thrombin inhibitor. Biochem Biophys Res Commun 1997; 235: 259–263
- Hurst JK, Barrette WC. Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol 1989; 24: 271–328
- Ramos CL, Pou S, Britigan BE, Cohen MS, Rosen GM. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J Biol Chem 1992; 267: 8307–8312
- Steineck MJ, Khan AV, Karnovsky MJ. Intracellular singlet oxygen generation by phagocytosing neutrophils in response to particles coated with a chemical trap. J Biol Chem 1992; 267: 13425–13433
- Ruzicka T, Printz MP. Arachidonic acid metabolism in skin: A review. Rev Physiol Biochem Pharmacol 1984; 100: 121–160
- Bonta JL, Parnham MJ. Prostaglandins and chronic inflammation. Biochem Pharmacol 1978; 27: 1611–1623
- Samuelsson B. Leukotriens: Mediators of immediate hypersensitivity reactions and inflammation. Science 1983; 220: 568–578
- Dhawan D, Balasubramanian S, Amonkar AJ, Singh N. Chemopreventive effect of 4′-demethyl epipodophyllotoxin on DMBA/TPA-induced mouse skin carcinogenesis. Carcinogenesis 1999; 20: 997–1003
- Wilkinson J, IV, Clapper M. Detoxification enzymes and chemoprevention. Proc Soc Exp Biol Med 1997; 216: 192–200
- Maliakal PP, Coville PF, Wanwimolruk S. Tea consumption modulates hepatic drug metabolizing enzymes in wistar rats. J Pharm Pharmacol 2001; 569–577
- Pegg AE, Shantz LM, Colemn CS. Ornithine decarboxylase as a target for chemoprevention. J Cell Biochem Suppl 1995; 22: 132–138