Abstract
Enzyme inhibition by fullerene derivatives has attracted much attention. In this communication, effects of two water-solube fullerene derivatives, fullerol and trimalonic acid C60 (TMA C60) on polymerase chain reaction (PCR) were investigated by using PCR of β-actin cDNA derived from HeLa cells as an experimental model. Both fullerol and TMA C60 were found to inhibit PCR in a dose-dependent manner. PCR was ultimately inhibited while the concentrations of each compound were not less than 0.01 mM. In contrast, mannitol exerted no effects on PCR while its concentration increased up to 2 mM. Compensation experiments with Thermus aquaticus (Taq) DNA polymerase revealed that both fullerol and TMA C60 inhibited the enzymatic activity of Taq DNA polymerase, and the inhibitory potency of TMA C60 was slightly greater than that of fullerol. Our data provides some novel aspects on the enzyme inhibiting activities of fullerene derivatives.
Introduction
To explore the biomedical application of fullerenes is currently an active research subject. During the last decade, many effective techniques for preparing water-soluble fullerenes and their derivatives have been reported, and an increasing number of papers have been published on different biological activities of fullerenes including DNA cleavage, antibacterial and antiviral, cytotoxicity, as well as antiapoptosis Citation1-7. Especially, fullerene derivatives could inhibit the enzymatic activities of several interesting enzymes, mainly consisting of oxidation-reduction related enzymes such as glutathione transferase, glutathione reductase and nitric oxide synthase, and viral enzymes such as HIV protease (HIVP), HIV reverse transcriptase (HIV-RT) and hepatitis C virus RNA-dependent RNA polymerase (HCV-RP) [Citation2,Citation8].
The polymerase chain reaction (PCR) is an important technique extensively used for in vitro amplification of DNA fragments, and has been applied in many aspects of biomedical research. The key component within a PCR system is the thermophilic deoxyribonucleic acid polymerase (DNA polymerase) [Citation9,Citation10]. Here we present evidence for the first time on the inhibitory activity of fullerene derivatives against a thermophilic DNA polymerase, Thermus aquaticus (Taq) DNA polymerase (EC 2.7.7.7).
Materials and methods
Two water-soluble fullerene derivatives, fullerol and trimalonic acid C60 (TMA C60) (see ) were synthesized according to previous literature [Citation11,Citation12]. Effects of fullerene derivatives dissolved in deionized water on PCR were investigated using the PCR of β-actin cDNA derived from human cervix uteri tumor-derived HeLa cells as an experimental model. β-actin, a component of cytoskeletal proteins in eukaryotic cells, is often used as an internal control in reverse transcription (RT)-PCR analysis to ensure that the same amount of each RNA sample was tested [Citation13]. HeLa cells were cultured with DMEM (Gibco, USA) supplemented with 15% activated fetal bovine serum (TBD, China), 100 U/mL penicillin and 100 μg/mL streptomycin in an incubator (Precision Scientific, USA) providing a moisturized atmosphere of 37°C and 5% CO2. After cells were lyzed with Trizol reagent (Invitrogen Co., USA), total RNA was isolated by the chloroform-isopropanol protocol, and dissolved in DEPC treated water. Synthesis of cDNA was carried out with 5 μg total RNA among 20 μL RT reaction. Then β-actin primers (forward: 5’ → 3’ AAGGATTCCTATGTGGGC; reverse: 5’ → 3’ CATCTCTTGCTCGAAGTC), Taq DNA polymerase (EC 2.7.7.7, Dingguo China) and 2.5 μL cDNA template were mixed together in a total volume of 50 μL, and PCR was performed under a model 9700 thermal cycler (PE, USA). The parameters set for PCR were as follows: 94°C/3 min; 94°C/50 s, 57°C/50 s, 72°C/30 s, 30 cycles; 72°C/7 min. The final product with a size of 534 bp was detected by 1.8% agarose gel electrophoresis and visualized on a ChampGel-3200 gel image analyzer.
Results and discussion
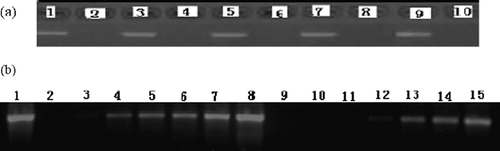
Agarose gel electrophoresis showed a single band of 534 bp corresponding to the PCR product of β-actin cDNA (, lane 2), with DNA fragment size indicated by a standard DNA marker (, lane 1). To the PCR mixture was added 1 mM, 0.1 mM, 0.06 mM, 0.03 mM, 0.01 mM, 0.005 mM and 0.001 mM of fullerol (, lane 3 ∼ 9) or TMA C60 (, lane 10 ∼ 16). The results showed that the PCR product decreased with the increase in the concentrations of both compounds, and became invisible on the gel when the concentrations of fullerol and TMA C60 were not less than 0.01 mM and 0.005 mM, respectively (, lane 3 ∼ 7; 10 ∼ 15). These data indicated an inhibitory activity of fullerol and TMA C60 on PCR amplification of β-actin cDNA. The effects of mannitol (, lane 1 ∼ 5) and DMSO (, lane 1, 6 ∼ 9) on PCR were also examined. The gradient concentrations of both chemicals included 0.1 mM, 0.5 mM, 1 mM and 2 mM. It was found that 2 mM DMSO induced disappearance of the PCR product (, lane 9), in accordance with the fact that DMSO was an inhibitor of Taq DNA polymerase [Citation14]. In contrast, mannitol had no effect on PCR at all tested doses. Although the structures of mannitol, fullerol and TMA C60 are quite different, one similarity among them is that they all have many hydroxyl groups. The fact that both fullerene derivatives but not mannitol can inhibit PCR of β-actin cDNA, implied that not only a number of hydroxyl groups, but other factors included in the structures of the fullerene derivatives, were probably responsible for their effects on Taq DNA polymerase.
Figure 2 A typical result from duplicate experiments showing the PCR of β-actin cDNA derived from HeLa cells in the presence of fullerol (lane 3 ∼ 9) or TMA C60 (lane 10 ∼ 16). Lane 1: standard DNA marker; lane 2: without fullerene derivative; lane 3,10: 1 mM; lane 4,11: 0.1 mM; lane 5,12: 0.06 mM; lane 6,13: 0.03 mM; lane 7,14: 0.01 mM; lane 8,15: 0.005 mM; lane 9,16: 0.001 mM.

Figure 3 A typical result from duplicate experiments showing the PCR of β-actin cDNA derived from HeLa cells in the presence of mannitol (lane 1 ∼ 5) and DMSO (lane 1, 6 ∼ 9). Lane 1: normal PCR; lane 2,6: 0.1 mM; lane 3,7: 0.5 mM; lane 4,8: 1 mM; lane 5,9: 2 mM.

The above PCR process is actually an in vitro DNA synthesis catalyzed by a thermostable DNA polymerase, Taq DNA polymerase, while using β-actin cDNA as the template. In order to elucidate the target of fullerene derivative during this process, compensation experiments of both cDNA template and Taq DNA polymerase were performed. Different amounts of cDNA template ranging from 2.5 μL to 10.0 μL () or Taq DNA polymerase ranging from 3.8 U to 15.2 U () were added to the PCR mixture. The results showed that increasing amounts of cDNA template induced little changes in PCR product, either in the absence (, lane 1,3,5,7,9) or presence (, lane 2,4,6,8,10) of 0.01 mM fullerol. It was worthy of noting that increasing the amounts of cDNA template up to 4 times the initial dose was insufficient to reverse the inhibitory effect of fullerol (, lane 10). However, the case of Taq DNA polymerase was quite different. PCR product began to disappear after addition of 0.01 mM fullerol or TMA C60 at the initial enzyme amount of 3.8 U (, lane 2,9), but it partially recovered when the enzyme amount increased (, lane 3 ∼ 8; 10 ∼ 15). Furthermore, the enzyme amount causing the recovery of PCR product varied with different fullerene derivatives. The PCR product appeared again on the gel when the enzyme amount was no less than 7.6 U (, lane 4 ∼ 8) and 11.4 U (, lane 13 ∼ 15) in the presence of fullerol and TMA C60, respectively. These data indicated that fullerol could inhibit PCR by inactivating the enzymatic activity of Taq DNA polymerase, but not by affecting the oligonucleotide components like cDNA template. It was concluded that the target of fullerol in the PCR mixtures was Taq DNA polymerase. An inhibitory effect of TMA C60 on Taq DNA polymerase activity was also found, and it seemed a little greater than that of fullerol, since larger enzyme amounts needed to reverse the inhibition of the former than the latter.
Figure 4 A typical result from duplicate experiments showing different amounts of cDNA template (a) or Taq DNA polymerase (b) on the inhibitory activity of 0.01 mM fullerol (a,b) or TMA C60 (b) against PCR of β-actin cDNA derived from HeLa cells. a, different volumes of cDNA template were 2.5 μL (lane 1,2), 4.0 μL (lane 3,4), 6.0 μL (lane 5,6), 8.0 μL (lane 7,8) and 10.0 μL (lane 9,10), in the absence (lane 1,3,5,7,9) or presence of fullerol (2,4,6,8,10). b, different amounts of Taq DNA polymerase were 3.8 U, 5.7 U, 7.6 U, 9.5 U, 11.4 U, 13.3 U and 15.2 U in the presence of fullerol (lane 2 ∼ 8) or TMA C60 (lane 9 ∼ 15), while lane 1 represented a standard PCR with 3.8 U Taq DNA polymerase and without either fullerol or TMA C60.
Inhibition of HIV protease (HIVP) was first reported among the various biological activities of fullerenes [Citation3]. Fullerene derivatives were subsequently found to inhibit some other interesting enzymes, including HIV-RT and HCV-RP [Citation8]. HIVP, HIV-RP and HCV-RP are essential for viral survival and replication, thus fullerenes were regarded as new potential drugs applicable to treat HIV and HCV viruses [Citation3,Citation8]. The present report revealed for the first time that the fullerene derivatives fullerol and TMA C60 were able to inhibit the in vitro enzymatic activity of Taq DNA polymerase, a DNA-dependent DNA polymerase originating from a specific bacterium, the extreme thermophile T. aquaticus [Citation15]. Considering that fullerene derivatives have been regarded as potential candidates for novel antibacterial reagents [Citation1,Citation2], the exact significance of the present finding need to further clarification in future studies. For example, it is worthy of investigating whether the activities of other enzymes involved in the metabolisms of nucleic acids in bacteria and viruses could be affected by fullerene derivatives. In addition, it was discovered that the inhibition by TMA C60 was slightly greater than that of fullerol, suggesting a possible structure-function effect involved in the inhibitory mechanism. Ongoing research in our laboratory is currently undertaking the investigation of various fullerene derivatives with different kinds and numbers of adduct groups on the parent fullerene cage.
Conclusions
This report shows that PCR of β-actin cDNA, not β-actin cDNA itself, was affected by the fullerene derivatives, fullerol and TMA C60. The target for both fullerene derivatives was the enzyme for PCR, i.e., Taq DNA polymerase. Furthermore, the inhibitory effect of TMA C60 was a litter stronger than that of fullerol.
Acknowledgements
This work was supported by Specialized Research Fund for the Doctoral Program of Higher Education (No. 20030007011) and Basic Research Foundation of Beijing Institute of Technology (No. 200306C02).
References
- Pantarotto D, Tagmatarchis N, Bianco A, Prato M. Synthesis and biological properties of fullerene-containing amino acids and peptides. Mini-rev Med Chem. 2004; 4: 805–814
- Bosi S, Da Ros T, Spalluto G, Prato M. Fullerene derivatives: An attractive tool for biological applications. Eur J Med Chem 2003; 38: 913–923
- Friedman SH, Decamp DL, Sijbesma RP, Srdanov G, Wudl F, Kenyon GL. Inhibition of the HIV-1 protease by fullerene derivatives: Model building studies and experimental verification. J Am Chem Soc 1993; 115: 6506–6509
- Tatiana DR, Prato M, Novello F, Maggini M, Banfi E. Easy access to water-soluble fullerene derivatives via 1,3-dipolar cycloadditions of azomethine ylides to C60. J Org Chem 1996; 61: 9070–9072
- Cheng FY, Yang XL, Fan CH, Zhu HS. Organophosphorus chemistry of fullerene: Synthesis and biological effects of organophosphorus compounds of C60. Tetrahedron 2001; 57: 7331–7335
- Yang XL, Fan CH, Zhu HS. Photo-induced cytotoxicity of malonic acid of [60]fullerene derivatives and its mechanism. Tox Vitro 2002; 16: 41–46
- Tagmatarchis N, Shinohara H. Fullerenes in medicinal chemistry and their biological applications. Mini-rev Med Chem 2001; 1: 339–348
- Mashino T, Shimotohno K, Ikegami N, Nishikawa D, Okuda K, Takahashi K, Nakamura S, Mochizuki M. Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg Med Chem Lett 2005; 15: 1107–1109
- Mullis KB, Faloona FA. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol 1987; 155: 335–350
- Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988; 239: 487–491
- Li TB, Huang KX, Li XH, Jiang HY, Li J. Studies on the rapid preparation of fullerols and its formation mechanism. Chem J Chinese U 1998; 19: 858–860
- Cheng FY, Yang XL, Zhu HS. Synthesis of oligoadducts of malonic acid C60 and their scavenging effects on hydroxyl radical. J Phys Chem S 2000; 61: 1145–1148
- Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E. Housekeeping genes as internal standards: Use and limits. J Biotechnol 1999; 75: 291–295
- Hermann D, Foernzler D. Specific amplification of difficult PCR products from small amounts of DNA using FastStart Taq DNA Polymerase. Biochemica 2002; 4: 25–26
- Chien A, Edgar DB, Trela JM. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol 1976; 127: 1550–1557