Abstract
The enzyme carbonic anhydrase (E.C. 4.2.1.1) has a stimulatory effect on glaucoma, an eye disease that has a risk to dogs, which are models for the human eye disease, that is similar to that in humans.
In this study, some sulfonamide derivatives, 2-(3-cyclohexene-1-carbamido)-1,3,4-thiadiazole-5-sulfonamide (CCTS), 4-(3-cyclohexene-1-carbamido) methyl-benzenesulfonamide (CCBS), 2-(9-octadecenoylamido)-1,3,4-thiadiazole-5-sulfonamide (ODTS), 2-(4,7,10-trioxa-tetradecanoylamido)-1,3,4-thiadiazole-5-sulfonamide (TDTS), and 2-(8-methoxycoumarine-3-carbamido)-1,3,4-thiadiazole-5-sulfonamide (MCTS), as well as some anionic compounds (perchlorate and chloride) and existing medicines (dorzolamide-HCl, gentamicine sulphate, tropicamide, and procaine-HCl) were assayed for their inhibition of dog carbonic anhydrase (dCA), which was purified from erythrocytes on an affinity gel of L-tyrosine-sulfonamide-Sepharose 4B. ODTS showed the highest potency amongst the synthetic compounds with IC50 value 1.18 × 10− 5 M. Amongst the medicines tested, only dorzolamide showed inhibition with IC50 value 5.05 × 10− 4 M. Procaine and tropicamide actually showed an activatory effect, whereas gentamicine sulfate had no significant effect. The inhibitory effects of anionic compounds such as perchlorate and chloride were also investigated; whereas perchlorate showed inhibition, chloride did not.
Introduction
The term “glaucoma” has been simply defined as the process of ocular tissue destruction caused by a sustained elevation of the intraocular pressure (IOP) above its normal physiological limits [Citation1]. It is the specific effect of that elevated pressure upon the composite parts of the optic nerve that renders glaucoma an emergency. The existence of “normal tension” and “low tension” glaucomas in man has blurred this simple definition, for these diagnoses have their origin in the clinical similarities of the optic nerve degeneration seen with elevated IOP and distinct, non-pressure related factors such as disc ischaemia or retinal excitotoxicity. It has even be argued that the rise in IOP seen in primary open-angle glaucoma in man is an effect rather than the cause, with only the effect being assessed and treated by current therapies. Open-angle glaucoma has limited incidence in domesticated animal species, and we are rarely in a position to diagnose its early presence and thus inhibit ganglion cell degeneration early in the process. There is evidence to indicate that abnormality in ganglion cell function exists in beagle dogs with inherited primary open-angle glaucoma, before the elevation in IOP occurs. There is thus a strong temptation to use this evidence to suggest that the IOP changes themselves are purely a secondary feature to another, as yet ill-defined, disease process. Approximately one-half of one per cent of all dogs in the United States develop this problem. It is much rarer in cats. In animals and man, IOP increases because the normal channels through which fluid leaves the eye become obstructed [Citation2].
Two forms of glaucoma are recognized: primary and secondary. Primary glaucoma is due to an inherited abnormal angle at the point where the iris meets the cornea. This abnormal angle obstructs the exit of fluid from the eye. Because it is genetic, the second eye often becomes affected within 6–12 months of the first. Middle-aged and older dogs, as well as female dogs, are most at risk of primary glaucoma. While human primary glaucoma is termed open angle glaucoma (beagle dogs also get this), canine primary glaucoma is usually closed angle. Dog breeds commonly affected with closed angle glaucoma are Spaniels, Toy Poodles, Boston Terriers, Dalmatians, Basset Hounds and Huskies. Persian cats are the most susceptible cat breeds to glaucoma. Glaucoma is not a disease that can be cured. Primary glaucoma has a genetic component; pets with this condition should not be bred. Secondary glaucoma usually affects only one eye. This is the most common form in cats. Secondary glaucoma occurs due to inflammation of structures within the eye and there are many causes for such inflammation; infectious or autoimmune disease, trauma or cancer. Scarring (synechia) and distortion at the corneal/iris angle impedes fluid exit; fluid pressure then increases as it does in primary glaucoma. Secondary glaucoma can also occur when the lens is jarred loose from its attachments or when it moves due to degenerative changes in its attachments [Citation3].
An early sign of glaucoma is enlargement of the blood vessels of the sclera, or whites of the eyes, along with impaired vision or blindness. At an early stage, blindness may be reversible. Once blindness is 3–5 days old it is usually irreversible. These eyes often show a bluish, diffuse cloudiness of the cornea and the pupils of these eyes are dilated (mydriasis) because light stops at a damaged retina and cannot reach the optic centers of the brain [Citation4].
The carbonic anhydrase (CA) enzyme (E.C. 4.2.1.1) is found in many tissues of the body including the eye and catalyzes the reversible reaction between the hydration of carbon dioxide and the dehydration of carbonic acid, that is vital in pH homeostasis and CO2 transport. In humans, CA exists as a number of isoenzymes, the most active being CA-II, found primarily in red blood cells (RBCs), but also in other tissues. Inhibition of CA in the ciliary processes of the eye decreases aqueous humor secretion, presumably by slowing the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport. The result is a therapeutic reduction in IOP [Citation5].
In this study, our goal was to investigate the inhibitory effects upon dog carbonic anhydrase(dCA) activity of various compounds orginally synthesized by our group as potential CA inhibitors[Citation6], in comparison with some well-known drugs used in the treatment of glaucoma as well as some common anion inhibitors. More potent inhibitors may be useful in the development of new approaches in glaucoma treatment.
Materials and methods
Chemicals
L-tyrosine and p-aminobenzenesulfonamide were from Merck Chem. Co (Milan/Italy). 2-(3-cyclohexene-1-carbamido)-1,3,4-thiadiazole-5-sulfonamide (CCTS); 4-(3-cyclohexene-1-carbamido) methyl-benzenesulfonamide (CCBS); 2-(9-octadecenoylamido)-1,3,4-thiadiazole-5-sulfonamide (ODTS); 2-(4,7,10-trioxa-tetradecanoylamido)-1,3,4-thiadiazole-5-sulfonamide (TDTS); and 2-(8-methoxycoumarine-3-carbamido)-1,3,4-thiadiazole-5-sulfonamide (MCTS) were prepared as previously described [Citation6]. All other chemicals were obtained from Sigma Chem. Co. (Milan / Italy) and were of analytical grade.
Preparation of hemolysate
Blood samples (25 mL), taken from 5 healthy dogs weighing approximately 15 kg each, were anticoagulated with ACD (Acid-citrate-dextrose), centrifuged at 1848 × g for 20 min at 4°C and the supernatant was removed. The packed RBCs were washed three times with 0.9% NaCI and then hemolysed in cold water. The ghosts and any intact cells were removed by centrifugation at 18924 × g for 25 min at 4°C, and the pH of the hemolysate was adjusted to 8.5 with solid Tris-base. The 25 mL hemolysate was applied to an affinity column containing L-tyrosine-sulfonamide-Sepharose-4B [Citation7,Citation8] equilibrated with 25 mM Tris–HCl / 0.1 M Na2SO4 (pH 8.5). The affinity gel was washed with 50 mL of 25 mM Tris–HCl / 22 mM Na2SO4 (pH 8.5). The dCA was then eluted with 0.1 M NaCH3COO / 0.5 M NaClO4 (pH 5.6); fractions of 3 mL were collected and their absorbance was measured at 280 nm.
Protein content determination
A quantitative protein determination was done on the pooled peak of eluted dCA using the Coomassie Brilliant Blue G-250 method [Citation9].
Enzyme assay
Carbonic anhydrase activity was measured by the Maren method [Citation10], which is based on determination of the time required for the pH of a standard solution to decrease from 10.0 to 7.4 due to CO2 hydration. The assay solution was 0.5 M Na2CO3 / 0.1M NaHCO3 (pH 10.0) and Phenol Red was added as the pH indicator. One unit of CA activity is defined as that amount of the enzyme that reduces by 50% the time of CO2 hydration measured in the absence of enzyme.
Inhibition studies
The inhibition of dCA activity by various sulfonamides (CCTS, CCBS, ODTS, TDTS, MCTS), drugs (dorzolamide, procain, tropicamide and gentamicine sulphate) and simple anions (perchlorate and chloride) were studied by determining their effects on the dCA-catalyzed CO2 hydration rate at 1°C. CO2 hydration rates were determined at five different inhibitor concentrations using an initial substrate concentration of 70 mM. Regression analysis was carried out on the graph of (percent inhibition) vs (inhibitor concentration). The inhibitor concentration which reduced enzyme activity by 50% (IC50), was then determined by interpolation.
Results and discussion
Inhibitors of carbonic anhydrase play an important role in ophthalmology, where they are used to reduce elevated IOP; this includes dorzolamide (Trusopt) and brinzolamide (Azopt) which are commonly used for glaucoma treatment [Citation11,Citation12]. In this study, our aim was to determine the inhibitory effects of some new compounds (i.e. CCTS, CCBS, ODTS, TDTS and MCTS) compared with Trusopt and a number of other drugs that are used in glaucoma treatment or generally in ophthalmology, i.e. Benoxinate (local anaesthesia) [Citation13], Tropamid (anticholinergy, midriatic) [Citation14], Gentagut (antibacterial) [Citation15]. All compounds were tested against the purified dog carbonic anhydrase enzyme.








![]()
![]()
Figure 1 Chemical structures of the substances tested for dCA inhibition.
In most mammalian species CA exhibits an unusual presence of more than one isoform, due to the presence in erythrocytes of two genetically distinct isozymes which differ strongly in specific activity, amino acid composition and immunological properties. Sciaky and Laurent indicated two forms of CA in dog erythrocytes which they designated as CA I for the low-activity form and CA II for the high-activity form [Citation16]. However, there are exceptions to this pattern in that there is an apparent absence of the low activity (CA I) form in the erythrocytes of some ruminants Citation17-21 and carnivores, including the dog and cat Citation22-24. Thus the true situation in dogs is rather confused.
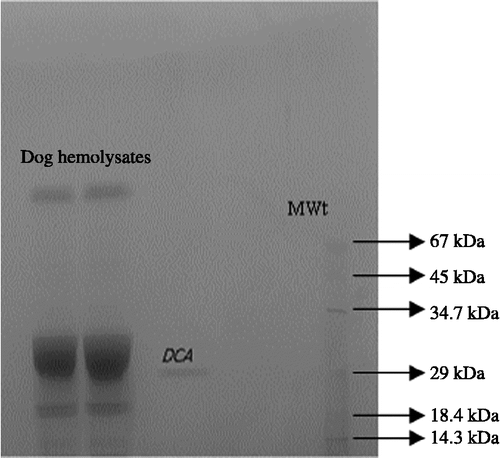
We have previously demonstrated the effectiveness of the immobilized ligand L-tyrosine-sulfonamide for the single-step affinity purification of CA from the erythrocytes of various freshwater and seawater fish [Citation7,Citation8]. In the present study, dCA was effectively purified from dog erythrocytes by one-step chromatography on L-tyrosine-sulfonamide coupled to Sepharose-4B (). A 452-fold purification with a yield of 29.7% was achieved (), and the mass and purity of the enzyme was assessed by SDS-PAGE (). The purified CA appeared as a single species with a mass of 29 kDa and apparent high activity, i.e. probably corresponding to CA II ().
Figure 1 The CO2-hydratase activity and protein concentration (280 nm absorbance) of dCA fractions eluted from affinity column with 0,1 M NaCH3COO / 0,5 M NaClO4 (pH5.6).
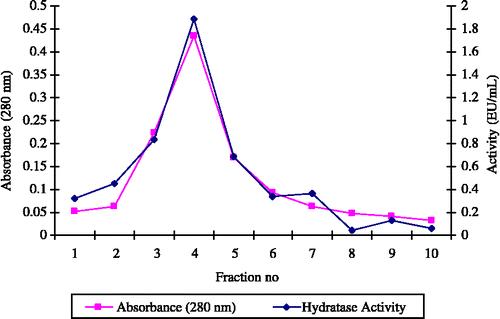
Table I. Purification of dCA by affinity chromatography.
Figure 2 SDS-PAGE of dCA on a 10% polyacrylamide reducing gel. Protein standards are: lysozyme (14.3 kDa), β-galactoglobulin (18.4 kDa), carbonic anhydrase (29 kDa), pepsin (34.7 kDa), ovalbumin (45 kDa), albumin bovine (BSA, 67 kDa).
We determined the inhibition constants for all the test compounds with respect to the CO2-hydratase activity of CA, which is the primary physiological function of this enzyme. The IC50 values are presented in . All the five new sulfonamide compounds demonstrated inhibitory activities against dCA. Comparing them, it is clear that the most potent inhibitor of dCA was ODTS with an IC50 value of 1.18 × 10− 5 M. In previous literature, Cakır and his colleagues had determined the IC50 values for ODTS acting upon the human CA isozymes, hCA-I and hCA-II, as being 0.61 × 10− 5 M and 0.52 × 10− 5 M, respectively [Citation6]. In that same study, the most potent inhibitor against the hCA-I and hCA-II isozymes was TDTS. But in the present work, a slightly poorer IC50 value of 1.59 × 10− 5 M was obtained with dCA. The other three sulfonamide derivatives tested. i.e. CCTS, CCBS and MCTS, were even less effective, yielding IC50 values with dCA of 2.57 × 10− 5 M, 2.40 × 10− 5 M and 2.53 × 10− 5 M, respectively (). Cakır's study had reported corresponding IC50 values for the same compounds acting upon hCA-I as being 0.43 × 10− 5, 0.55 × 10− 5 and 0.47 × 10− 5 M; and upon hCA-II as being 0.29 × 10− 5, 0,17 × 10− 5 and 0.96 × 10− 5 M, respectively [Citation6].
Table II. IC50 values for various compounds that inhibited dCA, ranked in decreasing effectiveness.
Trusopt, Benoxinate, Tropamid and the antibacterial drug Gentagut were also tested for their inhibitory effects upon dCA activity. Interestingly, the effective compounds that comprise these various drugs had varied effects upon dCA activity.
The classical CA inhibitors such as acetazolamide, dorzolamide and brinzolamide are used for glaucoma treatment [Citation25]. Here we showed that dorzolamide inhibited dCA activity with an IC50 of 5.05 × 10− 4 M, which is poorer than all five of the new sulfonamide compounds tested in parallel in this study. Previously this drug showed inhibitory activities against hCA-I, hCA-II and hCA-IV isozymes with IC50 values of 6 × 10− 7 M, 1.8 × 10− 10 M and 6.9 × 10− 9 M, respectively [Citation26]. However, Supuran's group obtained IC50 values for dorzolamide-HCl against hCA-I and hCA-II isozymes of 5.0 × 10− 5 and 9 × 10− 9, respectively [Citation27].
Surprisingly, both Benoxinate and Tropamid exhibited potentiation of dCA activity with increasing concentration, the effect being most marked with the former compound. In contrast, gentamicine showed a weak biphasic response. An initial weak inhibitory response at low concentrations (no IC50 was attained) was partially reversed at higher concentrations.
It is known that certain anions are potent inhibitors of carbonic anhydrase enzymes [Citation28]. For N3− ion at pH 5.8, Ki values were reported as being 1.5 × 10− 5 M and 0.2 × 10− 3 M for hCA-I and hCA-II, respectively. Also for the SCN− ion at pH 6.0, Ki values were 1.8 × 10− 5 M and 0.3 × 10− 3 M, and for the I− ion at pH 7.5,they were 0.7 × 10− 6 M and 8.7 × 10− 3 M for hCA-I and hCA-II, respectively Their mode of binding to the enzyme is known to be completely different from that of the sulfonamides. This effect is most marked against hCA-II versus hCA-I at low pH. For the Cl− anion at pH 6.5, Ki values of 0.004 M and 0.27 M, respectively, have been obtained; at pH 7.5 the corresponding values are 0.018 M and 0.73 M respectively [Citation28]. Other anions have also been shown to be inhibitory including perchlorate [Citation28]. Supuran and his group have determined IC50 values of 0.69 × 10− 3 and 1.26 × 10− 3 for perchlorate acting upon hCA-I and hCA-II, respectively [Citation29]. In the present study a similar IC50 of 1.07 × 10− 3 M was determined for perchlorate acting upon dCA. By comparison, chloride anion was only weakly inhibitory at the lowest concentrations and activity broadly recovered with increasing concentrations.
In conclusion, we show in this study that dog CA can be readily purified by a one-step affinity chromatography procedure for subsequent use in the evaluation of potential CA inhibitors. The susceptibilities of dCA to inhibition by the classical CA inhibitor, dorzolamide, the perchlorate anion, and some new sulfonamide compounds are comparable to the human CA isoforms. All the five new sulfonamide compounds tested show lower IC50 values than dorzolamide, with the most active, ODTS, being over 40-fold more potent. Interestingly, two other drugs used in ophthalmology apparently exhibit the ability, at the concentrations tested, to potentiate CA activity in these assays, by an, as yet, unknown mechanism. Hopefully, the levels of inhibition seen with the new sulfonamides will stimulate further drug development with the aim of generating more therapeutically-effective CA inhibitors for potential use in the treatment of glaucoma.
Acknowledgements
The authors would like to thank Balikesir University Research Center of Applied Sciences (BURCAS / Balikesir, Turkey) for providing the research facilities and also to Dr. Malcolm LYON, Senior Research Fellow in the Paterson Institute for Cancer Research University of Manchester for his invaluable contribution to this paper.
References
- Hoyng PFJ, Kitazawa Y. Medical treatment of normal tension glaucoma. Elsevier Science 2002; 9: 116–124
- Maren TH, Jankowska L, Sanyal G, Edelhauser HF. The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors and their effect on aqueous humor secretion. Exp Eye Res 1983; 36: 457
- Fiscella RG, Green A, Patuszynski DH, Wilensky J. Medical therapy cost considerations for glaucoma. Elsevier Science Inc 2003; 136: 18–25
- Barnebey H, Kwok YS. Patients' acceptance of a switch from dorzolamide to brinzolamide for the treatment of glaucoma in a clinical practice setting. Clin Ther 2000; 10: 1204–1212
- http://www.fda.gov/cder/foi/label/2002/20408S25lbl.pdf
- Çakır Ü, Uğraş Hİ, Özensoy Ö, Sinan S, Arslan O. In vitro inhibition effects of some new sulfonamide inhibitors on human carbonic anhydrase I and II. J Enz Inhib Med Chem 2004; 19(3)257–261
- Ozensoy O, Arslan O, Oznur Sinan S. A new method for purification of carbonic anhydrase isozymes by affinity chromatography. Biochemistry(Moscow) 2004; 69: 216–219
- Isık S, Kockar F, Ozensoy O, Arslan O. Fresenius Environ Bull 2004; 13(1)25–29
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye. Binding Anal Biochem 1976; 72: 248
- Maren TH. Carbonic anhydrase: General perspectives and advances in glaucoma research. Drug Dev Res 1987; 10: 255–276
- Vanlandingham BD, Brubaker RF. Combined effect of dorzolamide and latanoprost on the rate of aqueous humor flow. Elsevier Science Inc. 1998; 126: 191–196
- Fiscella RG, Green A, Patuszynsk DH. Wilensky J Medical therapy cost considerations for glaucoma American Journal of Ophthalmology 2003; 136(1)18–25(8)
- Schmid KL, Abbott M, Humphries M, Pyne K, Wildsoet CF. Timolol lowers intraocular pressure but does not inhibit the development of experimental myopia in chick. Exp Eye Res 2000; 70(5)659–666
- Manny RE, Hussein M, Scheiman M, Kurtz D, Niemann K, Zinzer K, and the COMET Study Group]. Tropicamide (1%): An Effective Cycloplegic Agent for Myopic Children Invest Ophthal Vis Sci 2001; 42(8)1728–1735
- http://www.animaleyecare.net/diseases/glaucoma.htm
- Sciaky M, Laurent G. Evidence for high and low activity carbonic anhydrases in the red cells of the dog. FEBS Letts 1976; 63(1)141–144
- Lindskog S. Purification and properties of bovine erythrocyte carbonic anhydrase. Biochim Biophys Acta 1960; 39: 218–226
- Cvek K, Dahlborn K, Ridderstraile Y. Localization of carbonic anhydrase in the goat mammary gland during involution and lactogenesis. J Dairy Res 1998; 65: 43–54
- Tanis RJ, Tashian RE. Purification and properties of carbonic anhydrase from sheep erythrocytes. Biochemistry 1971; 10(26)4852–4858
- Ashworth RB, Brewer JM, Stanford RL. Composition and carboxyl-terminal amino acid sequences of some mammalian erythrocyte carbonic anhydrases. Biochem Biophys Res Commun 1971; 44(3)667–674
- Ridderstraile Y, Persson E, Dantzer V, Leiser R. Carbonic Anhydrase Activity in Different Placenta Types: A Comparative Study of Pig, Horse, Cow, Mink, Rat, and Human Microsc Res Tech 1997; 38: 115–124
- Byvoet P. Gotti a isolation and properties of carbonic anhydrase from dog kidney and erythrocytes. Mol Pharmacol 1967; 3(2)142–152
- Soliman MH, Kluh I. Isolation of carbonic anhydrase from dog erythrocytes and determination of its N-terminal amino-acid sequence. Eur J Biochem 1974; 44: 611–615
- Tanis RJ, Tashian RE. Purification and Properties of Carbonic Anhydrase from Sheep Erythrocytes Biochemistry 1971; 10(26)4852–4856
- Sugrue MF. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors Elsevier Science 2000; 19: 87–112(26)
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors. Curr Med Chem-Imm Endoc Metab Agents. 2001; 1: 61–97
- Supuran CT, Casini A, Scozzafava A. Carbonic anhydrase inhibitors. Med Res Rev 2003; 23: 146–189
- Arslan O. Ph D Tesis. Atatürk Üniversitesi, Fen Bilimleri Enstitüsü Kimya. Erzurum, Anabilim Dalı 1994
- Cecchi A, Taylor SD, Yong L, Hill B, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of the human isozymes I, II, VA, and IX with a library of substituted difluoromethanesulfonamides. Bioorg Med Chem Lett 2005; 15(23)5192–5196