Abstract
The potency of newly developed oximes (K074, K075) and commonly used oximes (obidoxime, HI-6) to reactivate nerve agent-inhibited acetylcholinesterase was evaluated in rats poisoned with tabun or cyclosarin at a lethal dose corresponding to the LD50 value. In vivo determined percentage of reactivation of tabun-inhibited blood and brain acetylcholinesterase showed that obidoxime is the most efficacious reactivator of tabun-inhibited acetylcholinesterase among studied oximes in the peripheral compartment (blood) although the differences between obidoxime and newly developed oximes were not significant. On the other hand, one of the newly developed oximes (K074) seems to be a significantly more efficacious reactivator of tabun-inhibited acetylcholinesterase in the central compartment (brain) than the other studied oximes. In addition, the oxime HI-6 is unable to sufficiently reactivate tabun-inhibited acetylcholinesterase in rats. In vivo determined percentage of reactivation of cyclosarin-inhibited blood and brain acetylcholinesterase in poisoned rats showed that HI-6 is the most efficacious reactivator of cyclosarin-inhibited acetylcholinesterase among the studied oximes in the peripheral (blood) as well as central (brain) compartment although the differences between the oxime HI-6 and other tested oximes in the brain were not significant.
Due to their reactivating effects, both newly developed K-oximes can be considered to be promising oximes for the antidotal treatment of acute tabun poisoning while the oximes HI-6 is still the most promising oxime for the treatment of acute cyclosarin poisonings due to its high potency in reactivating cyclosarin-inhibited acetylcholinesterase in the peripheral as well as central compartment.
Introduction
Highly toxic organophosphorus compounds called nerve agents are considered to be the most dangerous chemical warfare agents. The most important representatives of nerve agents are tabun, sarin, soman, cyclosarin and VX. They pose a potential neurotoxic threat to both military and civilian populations as evidenced in terroristic attacks in Japan [Citation1,Citation2]. Their acute toxic effects is based on phosphorylation of acetylcholinesterase (AChE, EC 3.1.1.7) leading to the irreversible inhibition of this enzyme and subsequent overstimulation of postsynaptic cholinergic receptors due to the accumulation of the neurotransmitter acetylcholine in synapses of the central and peripheral nervous systems [Citation3,Citation4].
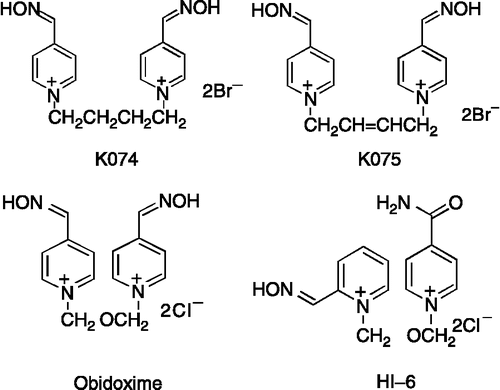
The medical countermeasures of nerve agent poisonings include the administration of special medicaments (antidotes) that are able to counteract the main toxic effects of nerve agents. The current standard antidotal treatment for poisoning with nerve agents usually includes a muscarinic cholinergic receptor antagonist to block the overstimulation of cholinergic receptors by acetylcholine and an oxime to reactivate nerve agent-inhibited AChE [Citation4,Citation5,Citation6]. In the past, the compounds with an oximate anion that was bound to a pyridinium ring were discovered and considered as the compounds able to reactivate nerve agent-inhibited AChE by dephosphorylating the enzyme molecule and restoring its activity. Their reactivating potency is based on the nucleophilic activity of the oxime group [Citation7]. While most of them are sufficiently effective to reactivate sarin or VX-inhibited AChE, their potency to reactivate soman, cyclosarin or tabun-inhibited AChE is generally low [Citation5,Citation8,Citation9]. Therefore, the antidotal treatment of acute nerve agent poisoning still represents a serious problem and the development of new, more effective AChE reactivators to achieve a satisfactory, effective antidotal treatment of acute poisoning with nerve agents regardless of their chemical structure still represents a very important goal. For this reason, new reactivators of nerve agent-inhibited AChE – 1,4-bis(4-hydroxyiminomethylpyridinium) butane dibromide (K074) and (E)-1,4-bis(4-hydroxyiminomethylpyridinium) but-2-en dibromide (K075) have been synthesized () [Citation10] to increase the reactivating efficacy of antidotal treatment of poisoning with nerve agents that were shown to be resistant to conventional oxime therapy, such as tabun (O-ethyl-N,N-dimethyl phosphoramidocyanidate) or cyclosarin (cyclohexyl methylphosphonofluoridate) [Citation11,Citation12,Citation13,Citation14]. Although these reactivators has been previously synthesized [Citation15] their potency in reactivating tabun- or cyclosarin-inhibited AChE has never been tested in vivo.
The aim of this study was to evaluate the reactivating effects of currently available oximes (obidoxime, HI-6) and newly developed oximes (K074, K075) in combination with an anticholinergic drug atropine in tabun- or cyclosarin-poisoned rats.
2 Material and methods
Male albino Wistar rats (wt. = 180–220g) were purchased from Konarovice (Czech Republic). They were kept in an air-conditioned room and allowed access to standard food and tap water ad libitum. The rats were divided into groups of eight animals (N = 8). The experimental protocol was approved by the Ethics Committee of the Faculty of the Military Health Sciences of University of Defense, Czech Republic. Tabun and cyclosarin were obtained from the Military Technical Institute in Brno (Czech Republic) and were 95% pure. Their purity was assayed by acidimetric titration. Obidoxime, the oxime HI-6 and newly developed oximes (K074, K075) of 98.5% purity were synthesized at the Department of Toxicology of the Faculty of Military Health Sciences in Hradec Kralove (Czech Republic). Their purity was analysed using HPLC. All other drugs and chemicals of analytical grade were obtained commercially and used without further purification. All substances were administered intramuscularly (i.m.) at a volume of 1 mL/kg body weight (b.w.).
To evaluate the reactivating efficacy of the oximes, the rats were injected i.m. with either atropine (21 mg/kg) alone or atropine (21 mg/kg) in combination with one of the oximes studied in equimolar dose (50 μmol/kg) 5 min before intramuscular tabun-or cyclosarin-poisoning in rats. The control rats were administered i.m. with saline instead of nerve agents and antidotes at the same volume. The prophylactic administration of antidotes was used because this procedure is suitable for a mechanistic study that compares the reactivating efficacy of various oximes. The technique should give better results than the treatment of animals after poisoning and reduce the influence of aging of the nerve agent-AChE complex [Citation16]. Moreover, some oximes are planned to be used prophylactically in certain chemical warfare scenarios [Citation9]. The rats were decapitated and exsanguinated to obtain the blood 30 min following tabun-poisoning at a dose of 200 μg/kg (LD50) or cyclosarin-poisoning at a dose of 90 μg/kg (LD50). The brains were removed and homogenized in distilled water and AChE activity determined by a spectrophotometric method [Citation17]. The reactivation (%) was calculated using the AChE activity values: {1- [((saline) – (oxime + atropine))/((saline) – (atropine control))]} × 100 [Citation16]. The AChE activity was expressed as μkat/kg or L (μmol substrate hydrolyzed/kg wet tissue or L blood/s).
Statistical significance was determined by the use of Student's t-test and differences were considered significant when P < 0.05. Statistical evaluation was determined with the relevant computer programs [Citation18].
Results
Obidoxime was demonstrated to be the most efficient reactivator of tabun-inhibited AChE in blood. It was able to increase the activity of tabun-inhibited AChE in blood by more than 20%. Nevertheless, the differences between obidoxime and the newly developed oximes (K074, K075) were not significant. In addition, its potency to reactivate tabun-inhibited AChE in the brain was negligible (). On the other hand, the newly developed oxime K074 seems to be a significantly more efficient reactivator of tabun-inhibited AChE in brain than other studied oximes (P < 0.05) (). It was able to increase the activity of tabun-inhibited AChE in the brain by more than 80% while the other oximes showed a relatively low reactivation (0 − 13.9%).
Table I. Rate of reactivation of tabun-inhibited AChE by oximes in rat blood and brain in vivo.
On the other hand, the oxime HI-6 seems to be the most efficient reactivator of cyclosarin-inhibited AChE in the peripheral as well as central compartment. It was able to increase the activity of cyclosarin-inhibited AChE in blood by more than 70% and in brain by almost 50% (). While the differences between HI-6 and other studied oximes (obidoxime, K-oximes) in blood were significant (P < 0.05), they were relatively small in the brain of poisoned rats. The newly developed oxime K075 was a significantly more efficient reactivator of cyclosarin-inhibited AChE than K074 in blood (P < 0.05) but their potency to reactivate cyclosarin-inhibited AChE in the brain is similar ().
Table II. Rate of reactivation of cyclosarin-inhibited AChE by oximes in rat blood and brain in vivo.
Discussion
Tabun and cyclosarin belong to a group of highly toxic organophosphorous compounds misused as chemical warfare agents for military as well as terroristic purposes. They differ from many other highly toxic organophosphates by their chemical structure and by the fact that commonly used antidotes (atropine in combination with an oxime) are not able to sufficiently eliminate their acute toxic effects [Citation8,Citation9].
For this reason, new AChE reactivators able to satisfactorily reactivate AChE inhibited by tabun or cyclosarin should be developed. According to our previous results, the number of pyridinium rings, the position of the oxime group in the pyridinium ring and the number of methylene groups linking the chain between two quaternary pyridinium rings in the molecule of reactivators play an important role in the reactivating potency of oximes [Citation11,Citation19,Citation20]. Generally, bisquaternary oximes have a higher affinity towards both intact and inhibited AChE and, therefore, higher potency to reactivate nerve agent-inhibited AChE compared to monoquaternary oximes [Citation20]. Unfortunately, the other above mentioned structural features, especially the position of the oxime group in the pyridinium ring, depend on the chemical structure of the AChE reactivators. While the potency of reactivators to reactivate tabun-inhibited AChE with the oxime group in position-4 is higher compared to reactivators with the oxime group at other different positions [Citation11,Citation19,Citation21], AChE reactivators with the oxime group in position-2 are the best reactivators of cyclosarin-inhibited AChE [Citation22,Citation23,Citation24]. On the other hand, the number of aldoxime groups is not so important. The oxime HI-6 has only one oxime group but it is significantly more efficient at reactivating soman- and cyclosarin-inhibited AChE than bispyridinium oximes with two aldoxime groups such as obidoxime, methoxime or trimedoxime [Citation25,Citation26]. The chain linking the two quaternary nitrogens in bispyridinium oximes exerts a great effect on reactivating ability, although this part of the oxime reactivator molecule does not play any role in the dephosphorylation process. It is a major factor in influencing oxime access and reactivation rates. A tri- or tetra-carbon chain seems to be the most suitable to impart sufficient potency to the oximes to reactivate tabun- or cyclosarin-inhibited AChE [Citation11,Citation22,Citation27].
Our results correspond to the previously published structural requirements which have been defined based on the in vitro results [Citation10,Citation11,Citation21,Citation22,Citation24,Citation26]. The evaluation of the ability of oximes to reactivate nerve agent-inhibited AChE in rat blood and brain demonstrated the difference between the potency of tested the oximes to reactivate tabun- or cyclosarin-inhibited AChE. While bispyridinium oximes with the oxime groups in position-4 (obidoxime, K oximes) are able to reactivate tabun-inhibited AChE at least in the peripheral compartment, the oxime HI-6 with the oxime group in position-2 showed negligible potency in reactivating tabun-inhibited AChE in blood and brain. On the other hand, the oxime HI-6 seems to be the most efficient reactivator of cyclosarin-inhibited AChE at the peripheral as well as the central compartment among all the oximes tested.
In conclusion, our data corresponding to in vitro results [Citation10,Citation24] confirm that there is no single, broad-spectrum oxime suitable for the antidotal treatment of poisoning with all organophosphorus agents [Citation8]. Both newly developed oximes (K074, K075) seem to be promising reactivators of tabun-inhibited AChE because they are able to significantly reactivate tabun-inhibited AChE at peripheral as well as central compartments, nevertheless, the differencies in reactivating efficacy between newly developed oximes (K074, K075) and some currently available oximes (obidoxime) is not so high to consider them as replacements for the currently used oximes in the treatment of acute tabun poisonings. On the contrary, the oxime HI-6, ineffective against tabun, is the most potent oxime for the reactivation of cyclosarin-inhibited AChE and, therefore, it is the most promising oxime for the antidotal treatment of acute cyclosarin poisoning.
Acknowledgements
The authors would like to thank to Mrs. M. Hrabinova and Mrs. J. Uhlirova for their skilful technical assistance and to Mr V. Blaha for the statistical data evaluation. The study was supported by the grant of Ministry of Defence, FVZMO0000501.
References
- Tu AT. Chemical Terrorism: Horrors in Tokyo Subway and Matsumoto City. Alaken Inc. 2002.
- Okumura T, Hisaoka T, Yamada A, Ishimatsu S, Takasu N. The Tokyo subway sarin attack-lessons learned. Toxicol Appl Pharmacol 2004; 197: 171–171
- Lotti M. Organophosphorus compounds. Experimental and Clinical Neurotoxicology, PS Spencer, HH Schaumburg. Oxford University Press, New York 2000; 898–925
- Marrs TC. Organophosphate poisoning. Pharmacol Ther 1993; 58: 51–66
- Dawson RM. Review of oximes available for treatment of nerve agent poisoning. J Appl Toxicol 1994; 14: 317–331
- Taylor P. Anticholinesterase agents. The Pharmacological Basis of Therapeutics, JG Hardman, LE Limbird. McGraw Hill, New York 1996; 161–176
- Shih TM. Comparison of several oximes on reactivation of soman-inhibited blood, brain and tissue cholinesterase activity. Arch Toxicol 1993; 67: 637–646
- Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol 2002; 40: 803–816
- Bajgar J. Organophosphate/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 2004; 38: 151–216
- Kuca K, Cabal J, Musilek K, Jun D, Bajgar J. Effective bisquaternary reactivators of tabun-inhibited AChE. J Appl Toxicol 2005; 25: 491–495
- Kuca K, Kassa J. A comparison of the ability of a new bispyridinium oxime - 1-(hydroxyiminomethylpyridinium)-4-(carbamoylpyridinium) butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vitro methods. J Enz Inhib Med Chem 2003; 18: 529–535
- Kassa J, Cabal J. A comparison of the efficacy of acetylcholinesterase reactivators against cyclohexyl methylphosphonofluoridate (GF agent) by in vitro and in vivo methods. Pharmacol Toxicol 1999; 84: 41–45
- Kassa J, Bajgar J. Comparison of the efficacy of HI-6 and obidoxime against cyclohexyl methylphosphonofluoridate (GF) in rats. Hum Exp Toxicol 1995; 14: 923–928
- Koplovitz I, Steward J. A comparison of the efficacy of HI-6 and 2-PAM against soman, sarin and VX in the rabbit. Drug Chem Toxicol 1992; 15: 117–126
- Pang YP, Kollmeyer TM, Hong F, Lee JC, Hammond PI, Haugabouk SP, Brimijoin S. Rational design of alkylene-linked bis-pyridiniumaldoximes as improved acetylcholinesterase reactivators. Chem Biol 2003; 10: 491–502
- Clement JG, Hansen AS, Boulet CA. Efficacy of HLö-7 and pyrimidoxime as antidotes of nerve agent poisoning in mice. Arch Toxicol 1992; 66: 216–219
- Ellman GL, Courtney DK, Andres V Jr, Featherstone RM. A new and rapid determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88–93
- Tallarida R, Murray R. Manual of Pharmacological Calculation with Computer Programs. Springer-Verlag, New York 1987
- Cabal J, Kuca K, Kassa J. Specification of the structure of oximes able to reactivate tabun inhibited acetylcholinesterase. Pharmacol Toxicol 2004; 95: 81–86
- Kuca K, Jun D, Musilek K. Structural requirements of acetylcholinesterase reactivators. Mini Rev Med Chem 2006; 6: 269–277
- Kuca K, Cabal J, Kassa J. A comparison of the efficacy of a bispyridinium oxime - 1,4-bis-(2-hydroxyiminomethylpyridinium) butane dibromide and currently used oximes to reactivate sarin, tabun or cyclosarin-inhibited acetylcholinesterase by in vitro methods. Pharmazie 2004; 59: 795–798
- Kuca J, Patocka J. Reactivation of cyclosarin-inhibited rat brain acetylcholinesterase by pyridinium-oximes. J Enz Inhib Med Chem 2004; 19: 39–43
- Hrabinova M, Musilek K, Jun D, Kuca K. New group xylene linker containing acetylcholinesterase reactivators as antidotes against nerve agent cyclosarin. J Enz Inhib Med Chem 2006; 21(5)515–519
- Kuca K, Cabal J, Jun D, Bajgar J, Hrabinova M. Potency of new structurally different oximes to reactivate cyclosarin-inhibited human brain acetylcholinesterase. J Enz Inhib Med Chem 2006; 21(6), (in press)
- Kassa J, Cabal J. A comparison of the efficacy of a new asymmetric bispyridinium oxime BI-6 with currently available oximes and H oximes against soman by in vitro and in vivo methods. Toxicology 1999; 132: 111–118
- Kuca K, Cabal J, Jun D, Kassa J, Bartosova L, Kunesova G. In vitro reactivation potency of some acetylcholinesterase reactivators against sarin- and cyclosarin-induced inhibitions. J Appl Toxicol 2005; 25: 296–300
- Worek F, Widmann R, Knopff O, Szinicz L. Reactivating potency of obidoxime, pralidoxime, HI-6 and HLö-7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol 1998; 72: 237–243