Abstract
A series of new benzenesulfonamides, most of which are chiral, incorporating 1, 3, 4-oxadiazole and amino acid moieties have been synthesized. Some of these compounds were screened for antimalarial activity and also evaluated for their ability to inhibit hem polymerization. The electrophoretic analysis indicated that one compound was effective in inhibiting the degradation of hemoglobin. The synthesized compounds were tested in mice infected with Plasmodium berghei. These derivatives have the potential for the development of novel antimalarial lead compounds.
Introduction
Malaria is one of the most widespread infectious diseases in the world. The disease affects approximately 500 million people worldwide, causing 2.5 million people die annually, mainly children in African countries [Citation1]. Four protozoan species infect humans, with Plasmodium falciparum, the most virulent human malaria parasite, being responsible for the majority of deaths [Citation1].
Further complicating this grim scenario is the emergence of widespread resistance to the available antimalarial drugs accompanied by a worldwide resurgence of malaria, requiring the development of new drugs to combat this disease. Medium or long acting sulfonamides have been used clinically as antimalarial agents, particularly sulfadiazine and sulfadoxine. However each is much more effective when given in combination with pyrimethamine or trimethoprim [Citation2]. Apart from their previous and more recently published antimalarial activity reports [Citation3], sulfonamides have extensively been documented for their wide variety of pharmacological activities such as antimicrobial, insulin-releasing antidiabetic, carbonic anhydrase inhibitory, anti-HIV, high ceiling diuretic, antithyroid and antitumor Citation4-5. Sildenafil citrate, another sulfonamide derivative, was approved as the first drug for treating male erectile dysfunction (ED) [Citation6] and a second generation antimitotic sulfonamide, ER-34410, evolved again from a scaffold of antibacterial-like sulfonamides, being 2- to 3-fold more potent than E7010 in a panel of various human tumor cell lines in vitro, and unlike E7010, can be administered intravenously [Citation7,Citation8]. Therefore, the sulfonamide moiety is a crucial functionality because of its wide variety of pharmacological activities. In addition to various pharmacological applications of 2,5-disubstituted-1,3,4- oxadiazoles Citation9-13, the 2-phenyl -5- (trichloromethyl)-1,3,4-oxadiazole has been reported [Citation14] as a potent antimalarial agent.
Many benzenesulfonamide derivatives with biological activity also require a free –SH/mercaptoaryl group in order to show an enhanced antiviral or anti-carbonic anhydrase activity Citation15-17. Accordingly, our design and synthetic strategy (Scheme ) for the synthesis of novel chiral and achiral compounds incorporating sulfonamide, 1,3,4-oxadiazole, and free mercapto (–SH) entities in one molecule is based on these observations and hypotheses. Accordingly, the compounds designed and reported here have been evaluated for antimalarial activity.
Materials & methods
Chemistry
Experimental protocols
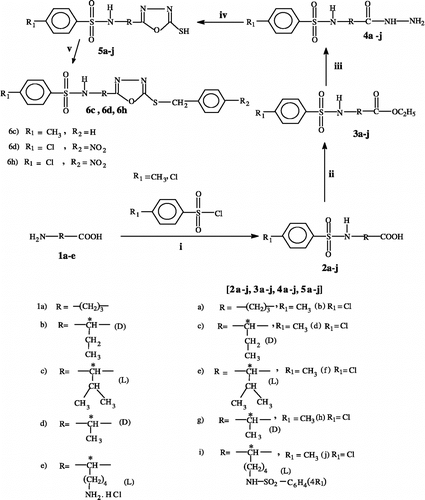
Melting points were determined on a Gallenkamp digital melting point apparatus and are uncorrected. Optical rotation data were recorded on a Perkin-Elmer 241 polarimeter. IR spectra were recorded in KBr disc on a FT-IR model FTS 3000 MX spectrometer. Elemental analysis was performed on a Carlo Erba 1106 elemental analyzer. 1H NMR (400 and 500 MHz) spectra were recorded on a Bruker NMR spectrophotometer. The chemical shifts of proton signals are in parts per million (ppm) downfield from tetramethylsilane (TMS) as an internal standard. EI-MS spectra were recorded on MAT 312 and MAT 311A mass spectrometers. Thin layer chromatography (TLC) was performed on precoated silica gel 60 F254 aluminum sheets (Merck). Intermediate compounds 2a–j of amino acids 1a–e and their corresponding esters 3a–j were prepared following an established literature procedure [Citation20a,b]. Compound 2e (yield 72%, m.p. 149°C, lit. 149°C) is has been reported in the literature [Citation20c].
A number of methods have been reported in the literature for the synthesis of benezenesulfonamides by using carboxylic acids/hydrazides [Citation18] and sulfonic acids/sulfonylchlorides [Citation19] as starting compounds. We report here a novel and cost-effective approach for preparing such compounds, using different chiral and achiral amino acids as starting material (Scheme ). The advantages of this method include ready availability of starting material, high yields of the final products (sulfonamides), a free mercapto group at position-5 of the oxadiazole ring, and most importantly the incorporation of chiral centre for the possible stereo-selectivity in the various applications of such compounds (e.g. as antimalarial agents). Benzyl and p-nitrobenzyl derivatives 6c, 6d and 6 h have been prepared from the corresponding free thiols 5c, 5d and 5h, using benzyl bromide and p-nitrobenzyl bromide (Scheme ). Amino acids 1a–e were converted to their corresponding sulfonamides 2a–j by reaction with 4-methylbenzenesulfonyl chloride or 4-chlorobenzenesulfonyl chloride in alkaline medium (Scheme ) using the standard literature method [Citation20a]. The carboxylic acid group of these sulfonamides 2a–j was esterified with ethanol in acidic medium [Citation20b], the esters 3a–j thus obtained were reacted with hydrazine hydrate (80%) to furnish the corresponding hydrazides 4a–j in good yields [Citation21]. The sulfonamides bearing the 2,5-disubstituted- 1, 3, 4-oxadiazole moiety, 5a–j, were then prepared by the reaction of the hydrazides 4a–j with carbon disulphide and potassium hydroxide [Citation22]. Mass spectroscopy (see experimental protocol) showed that the 2,5-disubstituted 1,3,4-oxadiazoles 5 are formed by the elimination of H2S from the carbohydrazides 4 and CS2. Structures of all the synthesized compounds were confirmed by micro analysis, optical rotation, IR, 1H-NMR and mass spectral data. The synthesis of compounds 4b, 4e, 4f, 4g, 5b, 5e, 5f and 5g has been reported earlier by our group.
General procedure for the synthesis of 4 – (4–methyl/chlorophenylsulfonamido)alkane hydrazide 4a–j
A mixture of 3 (10 mmole) and hydrazine hydrate (80%) in absolute ethanol (50 mL) was refluxed for 9h. The excess solvent was distilled off and residue was filtered, washed with water and recrystallized from 70% aqueous ethanol. Pure products 4a–j were collected in 71–89% yields.
4–(4 – Methylphenylsulfonamido)butane hydrazide 4a
Recrystallized from 70% aqueous ethanol, microcrystals, M.p. = 92–94 °C,Yield = 76%. IR (KBr)(ν, cm− 1) 3316, 3286 (NH), 1651 (C = O), 1365 (SO2), 1147 (SO2). 1H NMR (400 MHZ, Acetone-d6): δ = 1.65–1.72 (m, 2H, CH2), 2.5 (t, 2H, CH2, J = 7.4 Hz), 2.7 (t, 2H, CH2, J = 6.6 Hz), 2.39 (s, 3H, CH3), 7.35 (d, 2H, ArH, J = 8.0 Hz), 7.7 (d, 2H, ArH, J = 8.0 Hz), 9.3 (bs, 2H, NH2), 10.5 (bs, 1H, NH). EI-MS(%): 271(M+,2.4),198(20.6), 184 (12.2), 171 (6.9), 155 (66), 91 (100), 87 (4.2), 71 (2.1)
4– (4 – Chlorophenylsulfonamido)butane hydrazide 4b
Recrystallized from 70% aqueous ethanol, crystalline, M.p. 91–93°C, Yield = 75%. IR (KBr)(ν, cm− 1) 3377, 3285(NH), 1671(C = O), 1376 and 1155(SO2). 1H NMR (400 MHZ, Acetone-d6): δ 1.66–1.75(m, 2H, CH2), 2.55(t, 2H, CH2, J = 7.4 Hz), 2.73 (t, 2H, CH2, J = 6.7 Hz), 7.45(d, 2H, ArH, J = 8.0 Hz), 7.89(d, 2H, ArH, J = 8.0 Hz), 9.61(bs, 2H, NH), 11.29(bs, 1H, NH). EI-MS (%): 291 (M+, 19), 175(100), 111(87), 76(9), 57(11).
2– (4 – Methylphenylsulfonamido)butane hydrazide 4c
Recrystallized from 70% aqueous ethanol, microcrystals, M.p. = 146–148°C, Yield = 83%. IR (KBr)(ν, cm− 1) 3342, 3286 (NH), 1653 (C = O), 1361 (SO2), 1149 (SO2).1H NMR (400 MHZ, Acetone-d6): δ = 0.95 (dd, 3H, CH3, J = 7.4 Hz, J = 7.4 Hz), 1.81–1.89 (m, 2H, CH2), 2.37 (s, 3H, CH3), 4.42 (dd, H, CH, J = 7.5 Hz), 7.33 (d, 2H, ArH, J = 8.1 Hz), 7.76 (d, 2H, ArH, J = 8.0 Hz), 9.52 (bs, 2H, NH2), 10.70 (bs, 1H, NH). EI-MS (%): 271(M+, 12), 198 (23), 184 (11), 171 (6), 155 (69), 116 (4), 91 (100), 87 (5), 85 (3), 71 (4), 57 (9).
2– (4 – Chlorophenylsulfonamido)butane hydrazide 4d
Recrystallized from 70% aqueous ethanol, crystalline, M.p. = 155–157°C, Yield = 85%. IR (KBr)(ν, cm− 1) 3339, 3292(NH), 1653(C = O), 1365 and 1149(SO2). 1H NMR (400 MHZ, Acetone-d6): δ 0.97(dd, 3H, CH3, J = 7.2 Hz, J = 7.2 Hz), 1.81–1.90(m, 2H, CH2), 4.38(t, 1H, CH, J = 7.5 Hz), 7.65(d, 2H, ArH, J = 8.0 Hz), 8.03(d, 2H, ArH, J = 8.0 Hz), 9.76(bs, 2H, NH), 11.27(bs, 1H, NH). EI-MS (%): 291 (M+, 14), 175(25), 111(100), 76(19), 57(9).
2– (4 – Chlorolphenylsulfonamido)propane hydrazide 4h
Recrystallized from 70% aqueous ethanol, microcrystals, M.p. = 157–159°C, Yield = 89%. IR (KBr)(ν, cm− 1) 3375, 3281 (NH), 1671 (C = O), 1369 (SO2), 1165 (SO2). 1H NMR (400 MHZ, Acetone-d6): δ = 1.25 (d, 3H, CH3, J = 7.3 Hz), 4.73–4.81 (m, 1H, *CH), 7.55 (d, 2H, ArH, J = 8), 7.80 (d, 2H, ArH, J = 8 Hz), 9.12 (bs, 2H, NH2), 9.47 (bs, 1H, NH), 10.38 (bs, 1H, NH). EI-MS(%): 277 (M+, 4), 218 (100), 175 (95), 142 (14), 111 (69),102(3), 99 (33), 76 (3), 72 (33), 56 (5).
2,6-Bis(4-methylphenylsulfonamido]hexane hydrazide 4i
Recrystallized from 70% aqueous ethanol, microcrystals, M.p. = 125–127°C, Yield = 77%. IR (KBr)(ν, cm− 1) 3319, 3299 (NH), 1671 (C = O), 1341 (SO2), 1166 (SO2).1H NMR (400 MHZ, Acetone-d6): δ = 1.79–1.85 (m, 6H, 3CH2), 2.38 (s, 6H, 2CH3), 2.58 (td, 2H, CH2, J = 7.5 Hz, J = 4.1 Hz), 4.39 (dd, 1H, CH, J = 7.5 Hz, J = 7.4 Hz), 7.33 (d, 4H, ArH, J = 8.4 Hz), 7.67 (d, 4H, ArH, J = 8.4 Hz), 9.61 (bs, 2H, NH2), 10.53 (bs, 2H, NH). EI-MS (%): 313 (M+– 155, 4), 91 (100), Anal. Calc. for C20H28O5N4S2 (468.5878): C, 51.26; H, 6.02; N, 11.96; S, 13.68. Found: C, 51.03; H, 5.91; N, 11.93; S, 13.61%.
2,6-Bis(4-Chlorophenylsulfonamido]hexane hydrazide 4j
Recrystallized from 70% aqueous ethanol, microcrystals, M.p. = 135–137°C, Yield = 71%. IR (KBr)(ν, cm− 1) 3309, 3286 (NH), 1676 (C = O), 1375 (SO2), 1146 (SO2).1H NMR (400 MHZ, Acetone-d6): δ = 1.84–2.01 (m, 6H, 3CH2), 2.65 (td, 2H, CH2, J = 7.6 Hz, J = 4.1 Hz), 4.42 (dd, 1H, CH, J = 7.5 Hz, J = 7.6 Hz), 7.55 (d, 4H, ArH, J = 8.0 Hz), 7.91 (d, 4H, ArH, J = 8.0 Hz), 9.75 (bs, 2H, NH2), 11.21 (bs, 2H, NH). EI-MS (%): 334 (M+– 175, 3), 175 (100), Anal. Calc. for C18H22O5N4S2Cl2(509.424): C, 42.44; H, 4.35; N, 11.11; S, 12.59. Found: C, 42.47; H, 4.37; N, 11.31; S, 12.28%.
General procedure for the synthesis of 4-methyl/chloro-N-[3 or 1-(5-mercapto-1,3,4-oxadiazol-2-yl)alkyl]benzenesulfonamide 5a–j
The compound 4 (5.5 mmole) was dissolved in 80 mL absolute ethanol and 6.6 mmol of carbon disulphide was added followed by 5.5 mmol of potassium hydroxide dissolved in 10 mL of water. The reaction mixture was stirred for 15 min and then refluxed for 16.5 h. The progress of the reaction was monitored by TLC. The excess ethanol was distilled off and the reaction mixture was diluted with water and acidified with 4N HCl to pH 2–3 (Congo Red). The solid obtained was filtered, washed with water and recrystallized from 60% aqueous ethanol. Pure product 5a–j was collected in 80–91% yield.
4-Methyl-N-[(3-(5-mercapto-1,3,4-oxadiazol-2-yl)propyl]benzenesulfonamide 5a
Recrystallized from 60% aqueous ethanol, Microcrystals, M.p. = 145–147°C, Yield = 85%. IR (KBr)(ν, cm− 1): 3206 (NH), 2564 (SH), 1589 (C = N), 1379 (SO2), 1179 (SO2), 1285 (C = S). 1H NMR (400 MHZ, Acetone-d6): δ = 1.89–1.96 (m, 2H, CH2), 2.40 (s, 3H, CH3), 2.80 (t, 2H, CH2, J = 7.4 Hz), 3.04 (t, 2H, CH2, J = 6.6 Hz), 7.22 (bs, 1H, NH, exchangeable with D2O), 7.38 (d, 2H, ArH, J = 8.10 Hz), 7.72 (d, 2H, ArH, J = 8.2 Hz), 12.80 (bs,1H, NH + SH). EI-MS(%): 313(M+, 10), 240(7.2), 186(1.42), 155(49), 129(43), 91 (100), 65 (30). Anal. Calc. for C12H15O3N3S2 (313.388): C, 45.99; H, 4.82; N, 13.41; S, 20.46. Found: C, 45.71; H, 4.70; N, 13.22; S, 20.71%.
4-Chloro-N-[3-(5-mercapto-1,3,4-oxadiazol-2-yl)propyl]benzenesulfonamide 5b
Recrystallized from 60% aqueous ethanol, powder, M.p. = 171–172°C, Yield = 80%. IR (KBr)(ν, cm− 1) 3287( − NH), 2545( − C = N), 1375 and 1165( − SO2). 1H NMR (400 MHZ, Acetone-d6): δ1.92–1.98 (m, 2H, CH2), 2.80 (t, 2H, CH2, J = 7.5 Hz), 3.09 (t, 2H, CH2, J = 6.7 Hz), 7.25 (bs, 1H, NH, exchangeable with D2O), 7.63(d, 2H, ArH, J = 8.1 Hz), 7.87 (d, 2H, ArH, J = 8.2 Hz), 12.80 (bs,1H, NH + SH). EI-MS (%): 335(M++2), 333 (M+, 7), 260(14), 177(25), 175(69), 158(10), 141(13), 129 (91), 113(32), 112(10), 111(100), 98(24), 76(11), 75(48), 69(29), 56(22), 55(16). Anal. Calc. for C11H12O3N3S2Cl (333.7921): C, 39.58; H, 3.62; N, 12.59; S, 19.21. Found: C, 39.68; H, 3.74; N, 12.77; S, 19.20%.
4-Methyl-N-[1-(5-mercapto-1,3,4-oxadiazol-2-yl)propyl]benzenesulfonamide 5c
Recrystallized from 60% aqueous ethanol, crystalline, [α]D20 = +36.32 (C = 1.01 g/100 cm3, acetone). M.p. = 181–182 0C, Yield = 87%. IR (KBr)(ν, cm− 1) 3266 (NH), 2565 (SH), 1582 (C = N), 1356 (SO2), 1145 (SO2), 689 (C(S). 1H NMR (400 MHZ, Acetone-d6): δ = 0.92 (dd, 3H, CH3, J = 7.4 Hz, J = 7.4 Hz), 1.82-1.90 (m, 2H, CH2), 2.38 (s, 3H, CH3), 4.38 (dd, H, CH, J = 7.5 Hz, J 7.6 Hz), 7.21 (bs, 1H, NH, exchangeable with D2O), 7.32 (d, 2H, ArH, J = 8.1 Hz), 7.66 (d, 2H, ArH, J = 8.2 Hz), 12.75 (bs,1H, NH + SH). EI-MS(%): 313 (M+,11), 249(7), 212(9), 155(58), 147(5), 91(100), 65 (26). Anal. Calculated for C12H15O3N3O3S2 (313.388): C, 45.99; H, 4.82; N, 13.41; S, 20.46. Found: C, 45.81; H, 4.56; N, 13.26; S, 20.61%.
4-Chloro-N-[1-(5-mercapto-1,3,4-oxadiazol-2-yl)propyl]benzenesulfonamide 5d
Recrystallized from 60% aqueous ethanol, microcrystals, = +38.12 (C = 1.05 g/100 cm3, acetone). M.p. = 193–195 0C, Yield = 81%. IR (KBr)(ν, cm− 1) 3283( − NH), 2555( − SH), 1589( − C = N), 1365 and 1149( − SO2). 1H NMR (500 MHZ, Acetone-d6): δ 0.98 (t, 3H, CH3, J = 7.4 Hz), 1.82–1.91 (m, 2H, CH2), 4.39 (dd, H, CH, J = 7.8 Hz, J = 7.5 Hz,), 7.24 (bs, 1H, NH, exchangeable with D2O), 7.66 (d, 2H, ArH, J = 8.0 Hz), 7.87 (d, 2H, ArH, J = 8.0 Hz) 12.89(bs,1H, NH + SH). EI-MS (%): 333 (M+, 11), 260(4), 177(15), 175(67), 129(81), 111(100), 76(11), 69(21). Anal. Calcd for C11H12O3N3S2Cl (333.7921): C, 39.58; H, 3.62; N, 12.59; S, 19.21. Found: C, 39.57; H, 3.65; N, 12.20; S, 19.46%.
4-Chloro-N-[1-(5-mercapto-1,3,4-oxadiazol-2-yl)ethyl]benzenesulfonamide 5h
Recrystallized from 60% aqueous ethanol, powder, = +42.45 (C = 1.04 g/100 cm3, acetone) M.p. = 172–174 0C, Yield = 91%. IR (KBr)(ν, cm− 1) 3285( − NH), 2550( − SH), 1609( − C = N), 1356 and 1149( − SO2), 669 (C-S). 1H NMR (400 MHZ, Acetone-d6): δ 1.51 (d, 3H, CH3 J = 7.0 Hz), 4.65–4.73 (m, 1H, CH), 7.23 (bs, 1H, NH, exchangeable with D2O), 7.58 (d, 2H, ArH, J = 8.0 Hz), 7.83 (d, 2H, ArH, J = 8.0 Hz) 12.86(bs,1H, NH + SH). EI-MS (%): 319 (M+, 2), 244(10), 218(20), 177(39), 175(100), 144(38), 139(29) 111(87), 75(21). Anal. Calcd for C10H10O3N3S2Cl (319.779): C, 37.56; H, 3.15; N, 13.14; S, 20.05. Found: C, 37.74; H, 2.88; N, 13.10; S, 20.23%.
2-[1,5-Bis(4-methylphenylsulfonamido)]pentyl-5-mercapto-1,3,4-oxadiazole 5i
Recrystallized from 60% aqueous ethanol, Microcrystals, M.p. = 147–149°C, Yield = 89%. IR (KBr)(ν, cm− 1) 3299(NH), 1597(C = N), 1321and 1161(SO2). 1H NMR (400 MHZ, Acetone-d6): δ 1.37–1.48(m, 4H, 3CH2), 1.78(dd, 2H, CH2, J = 7.5 Hz, J = 7.5 Hz), 2.37(s, 3H, CH3), 2.41(s, 3H, CH3), 2.79(dd, 2H, CH2, J = 6.6 Hz, J = 6.6 Hz), 4.38(dd, 1H, CH, J = 7.5 Hz, J = 7.5 Hz), 7.21 (bs, 2H, NH, exchangeable with D2O), 7.32(d, 2H, ArH, J = 7.8 Hz), 7.37(d, 2H, ArH, J = 7.9 Hz), 7.65(d, 2H, ArH, J = 8.0 Hz), 7.74(d, 2H, ArH, J = 8.0 Hz), 12.85 (bs,1H, NH + SH). EI-MS (%) 381(11), 284(19), 170(10), 91(100), 76(9), 75(17). Anal. Calcd for C21H26O5N4S3 (510.662): C, 49.39; H, 5.13; N, 10.97; S, 17.84. Found: C, 49.28; H, 5.13; N, 11.27; S, 18.50%.
2-[1,5-Bis(4-chlorophenylsulfonamido)]pentyl-5-mercapto-1,3,4-oxadiazole 5j
Recrystallized from 60% aqueous ethanol, Microcrystals, M.p. = 153–155°C, Yield = 81%. IR (KBr)(ν, cm− 1) 3286(NH), 1607(C = N), 1327and 1165(SO2). 1H NMR (400 MHZ, Acetone-d6): δ 1.39–1.53(m, 4H, 3CH2), 1.75(dd, 2H, CH2, J = 7.5 Hz, J = 7.5 Hz), 2.79(dd, 2H, CH2, J = 6.6 Hz, J = 6.6 Hz), 4.39(dd, 1H, CH, J = 7.3 Hz, J = 7.3 Hz), 7.21 (bs, 2H, NH, exchangeable with D2O), 7.52(d, 2H, ArH, J = 7.8 Hz), 7.57(d, 2H, ArH, J = 7.9 Hz), 7.79(d, 2H, ArH, J = 8.0 Hz), 7.82(d, 2H, ArH, J = 8.0 Hz), 12.91 (bs,1H, NH + SH). EI-MS (%) 421(2), 324(11), 175(100), 111(81),76(15),75(7), Anal. Calcd for C19H20O5N4S3Cl2 (551.479): C, 41.38; H, 3.66; N, 10.16; S, 17.44 Found: C, 41.18; H, 3.69; N, 10.47; S, 17.30%.
General procedure for the synthesis of derivatives of 4-methyl/chloro-N-[1-(5-mercapto-1,3,4-oxadiazol-2-yl)alkyl]benzenesulfonamide (6c, 6d and 6h)
0.75 mmol (250 mg) of compound 5, 0.22 mmol (0.3 mL) of Et3N and a catalytic amount (25 mg) of DMAP were stirred in 25mL of dry CHCl3 for 15 min. 0.8 mmole of benzyl bromide/p-nitrobenzylbromide was added and the mixture was stirred for 5–7 h at 30–70°C. The reaction mixture was washed with dilute HCl, brine, water and dried over Na2 SO4 (anhydrous). The excess solvent was distilled off and product was recrystallized from 65% aqueous ethanol.
N-[1-(5-(benzylthio)-1,3,4-oxadiazol-2-yl)propyl]-4-methylbenzenesulfonamide 6c
Recrystallized from 65% aqueous ethanol, Prisms, = +24.52 (C = 0.54 g/100 cm3, acetone); M.p. = 113–1150C, Yield = 91%.IR (KBr)(ν, cm− 1) 3256( − NH), 1602( − C = N), 1345 and 1155( − SO2) 687(C − S).1H NMR (400 MHZ, Acetone-d6): δ 0.88 (dd, 3H, CH3, J = 7.5 Hz, J = 7.4 Hz), 1.75–1.80(m, 2H, CH2), 2.39(s, 3H,CH3), 4.17 (s, 2H, CH2), 4.28 (dd, H, CH, J = 7.8 Hz, J = 7.6 Hz,), 7.18 (bs, 1H, NH, exchangeable with D2O), 7.23–7.31 (m, 5H, ArH), 7.35 (d, 2H, ArH, J = 8.0 Hz), 7.67 (d, 2H, ArH, J = 8.4 Hz). EI-MS (%): 403 (M+, 19), 91(100). Anal. Calcd for C19H21O3N3S2 (403.515): C, 56.56; H, 5.25; N, 10.41; S, 15.89. Found: C, 56.36; H, 5.37; N, 10.38; S, 15.57%.
N-[1-(5-(4-nitrobenzylthio)-1,3,4-oxadiazol-2-yl)propyl]-4-chlorobenzenesulfonamide 6d
Recrystallized from 65% aqueous ethanol, Microcrystals, = +21.39 (C = 0.63 g/100 cm3, acetone); M.p = 116–117°C, Yield = 75%.IR (KBr)(ν, cm− 1) 3289( − NH), 1592( − C = N), 1371 and 1166( − SO2) 679(C–S). 1H NMR (400 MHZ, Acetone-d6): δ 1.51 (d, 3H, CH3 J = 7.0 Hz), 4.35 (s, 2H, CH2), 4.64–4.71(m, 1H, CH), 7.22 (bs, 1H, NH, exchangeable with D2O), 7.34 (d, 2H, ArH, J = 8.2 Hz), 7.56 (d, 2H, ArH, J = 8.4 Hz), 7.66 (d, 2H, ArH, J = 8.4 Hz), 8.17(d, 2H, ArH, J = 8.0 Hz). EI-MS (%): 454 (M+, 7), 175(100). Anal. Calcd for C17H15O5N4S2 Cl (454.904): C, 44.61; H, 3.32; N, 12.32; S, 14.11. Found: C, 44.56; H, 3.37; N, 12.68; S, 14.07%.
N-[1-(5-(4-nitrobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-chlorobenzenesulfonamide 6h
Recrystallized from 65% aqueous ethanol, Microcrystals, = +34.62 (C = 1.00 g/100 cm3, acetone); M.p. = 121–122°C, Yield = 79%.IR (KBr)(ν, cm− 1) 3286( − NH), 1595( − C = N), 1375 and 1166( − SO2), 689(C − S). 1H NMR (400 MHZ, Acetone − d6): δ 0.87 (dd, 3H, CH3 J = 7.4 Hz, J = 7.4 Hz), 1.79 − 1.84(m, 2H, CH2), 4.38 (s, 2H, CH2), 4.50 (dd, H, CH J = 7.6 Hz, J = 7.6 Hz,), 7.20 (bs, 1H, NH, exchangeable with D2O), 7.33 (d, 2H, ArH, J = 8.1 Hz), 7.54 (d, 2H, ArH, J = 8.2 Hz), 7.64 (d, 2H, ArH, J = 8.4 Hz), 8.14 (d, 2H, ArH, J = 8.4 Hz). EI-MS (%): 468 (M+, 11), 175(100). Anal. Calcd for C18H17O5N4S2 Cl (468.931): C, 46.10; H, 3.65; N, 11.95; S, 13.67. Found: C, 46.36; H, 3.38; N, 11.88; S, 13.29%.
Antimalarial assays
Parasite and experimental host
Male NIH mice, weighing 18–22 g were maintained on a commercial pellet diet and housed under conditions approved by the Ethics Committee. Plasmodium berghei (ANKA strain), a rodent malaria parasite, was used for infection. Mice were infected by i.p. passage of 1 × 106 infected erythrocytes diluted in phosphate buffered saline solution (PBS, 10 mM, pH 7.4, 0,1 mL). Parasitemia was monitored by microscopic examination of Giemsa stained smears.
Parasite extracts
Blood of infected animals, at a high level of parasitemia (30–50%), was collected by cardiac puncture with a heparinized syringe and the blood pool was centrifuged (500 × g × 10 min, 4°C). Plasma and buffy coats were removed and the red blood cells (RBC) pellet was washed twice with chilled PBS-Glucose (5.4%). The washed RBC pellet was centrifuged on a discontinuous percoll gradient (80–70% percoll in PBS-Glucose, 20000 × g × 30 min × 4°C) [Citation25]. The upper band (mature forms) was removed by aspiration, collected in Eppendorf tubes and washed twice with chilled PBS-Glucose and the infected erythrocytes were lysed with the non-ionic detergent saponin (0.1% in PBS × 10 min). Cold PBS (1 mL) was added and the samples were centrifuged (13000 × g × 5 min, 4°C) to remove erythrocyte cytoplasm content (including erythrocyte hemoglobin). The free parasites were mixed with PBS-Glucose (5.4%), and subjected to three freeze-thaw cycles ( − 70 °C /+37 °C). The final homogenate was used in the hemoglobin hydrolysis inhibition assay [Citation26].
Mice native hemoglobin
Native hemoglobin from non-infected mice was obtained by treating one volume of pellet erythrocytes with two volumes of water. The resulting solution was used as the substrate in the inhibition of the hemoglobin hydrolysis assay.
Inhibition of hemoglobin hydrolysis assay
The proteolytic effect of the parasite extract on the native mice hemoglobin was assayed using a 96-well tissue culture plate (Greiner Bio-One). The assay mixture contained: mice native hemoglobin (10 μL), parasite extract (50 μL), GSH (10 μL, 10 μM), and acetate buffer (0.2 M, pH 5.4) to a final volume of 100μL. The compounds (50 mM) were incorporated in the incubation mixture dissolved in DMSO. The incubations were carried out at 37 °C for 18 h and the reactions were stopped by addition of reduced sample buffer. The degree of digestion was evaluated electrophoretically by SDS-PAGE [Citation26] and the intact globin bands were analysed by densitometry. A DMSO control was electrophoresced at the same time.
4-Day suppressive test
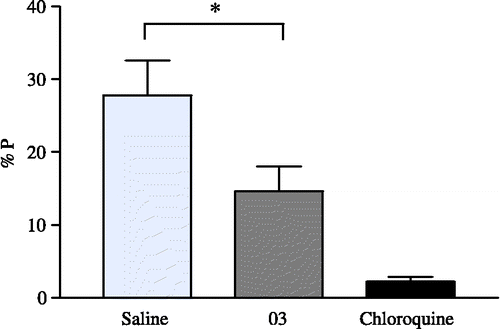
NIH mice (18–23 g) were infected i.p. (using caudal vein) with 106 infected red blood cells with Plasmodium berghei. Two hours after infection, treatment began with the best compounds tested in the hypoxanthine assay. These were dissolved in DMSO (0.1 M), diluted with Saline-Tween 20 solution (2%). Each compound (20 mg/kg) was administered once by i.p. for 4 days. At day four, the parasitemia was counted by examination of Giemsa stained smears. Chloroquine (25 mg/Kg) was used as a positive control. The survival time beyond the control group (without drug treatment) was recorded. The results were expressed as percentage of parasitaemia in relation to the control (% of parasitemia) and percentage of survival mice [Citation27].
Data analysis
Data were statistically analyzed using t-tests for specific group comparisons, assuming 95% of confidence according to Graph Pad Prism 3.02.
Inhibition of hem crystallization
The hem polymerization assay of compounds 4d, 4e, 4 h, 5a–i, 6c–d, and 6h was performed according to the reported procedure [Citation23]. The results are shown in . Briefly, a solution of haemin chloride (50 μL, 4 mM), dissolved in DMSO (5.2 mg/mL), was distributed in 96-well micro plates. Different concentrations (100-5 mM) of the compounds dissolved in DMSO, were added in triplicate in test wells (50 μL) with a final concentration of 25-1, 25 mM/well. Controls contained either water (50 μL) or DMSO (50 μL). β-Hematin formation was initiated by the addition of acetate buffer (100 μL 0.2 M, pH 4.4). Plates were incubated at 37°C for 48 h, carefully centrifuged for 15 min (4000 RPM, IEC-CENTRA, MP4R). After discarding the supernatant, the pellet was washed twice with 200 μL of DMSO and finally, dissolved in 200 μL of NaOH (0.2 N). The solublized aggregates were further diluted at a ratio of 1:2, with NaOH (0.1N). The absorbances were recorded at 405 nm (Microplate Reader, BIORAD-550). The results are expressed as a percentage of inhibition of flavoprotein (FP) polymerization. The results are reported in Table 1. Compound 4h showed a moderate decrease in the parasitaemia levels at 4th day post-infection, however, this did not cure the infected animals (). This could be due to bioavailability problems since this compound is not water-soluble. It is necessary to assay this drug with a different dose-regimen in further experiments.
Table I. Effects of compounds on the hem crystallization and hemoglobin proteolysis.
Results and discussion
To evaluate the antimalarial activity, we selected and tested the ability of the novel compounds 4d–e, 4h, 5a–i, 6c, 6d and 6h to inhibit hem polymerization, since hem can polymerize spontaneously in the food vacuole of the parasite under acid and low oxygen conditions [Citation23]. Therefore, each compound was tested for its inhibition of globins proteolysis, in an in vitro assay which uses a trophozoite-rich extract to digest the native hemoglobin of mice. The electrophoretic analysis indicated that within a total of 15 compounds studied, 4h was a potent inhibitor of the degradation of hemoglobin with an inhibition value of 54.14% (intact band at 14.4 kDa). Whereas, other compounds showed weak inhibition ( < 50%) due to the presence of some intact hemoglobin (Table 1). The active compound 4h (>50% inhibition of hemoglobin proteolysis), was tested in mice infected with Plasmodium berghei (ANKA strain, ), a chloroquine-susceptible strain of murine malaria. The mice were treated with compounds (chloroquine or the active ones, at 20 mg/kg, ip once daily) for 4 consecutive days (days 0–4 post infection, ), and their survival times and parasitaemias on the fourth day were monitored and compared with control mice receiving saline (untreated mice). Thus, chiral benzenesulfonamides and their derivatives, especially the compound 4h, reported here may be considered as very interesting lead molecules for the possible design of novel selective antimalarial drugs.
The structures of the synthesized compounds are supported by physical, optical rotation data (for chiral compounds), micro analytical data, IR, 1H NMR and mass spectral data. The IR spectrum of the representative sulfonamide 5h, revealed the presence of characteristic bands for -NH at 3285 cm− 1, and 2550 cm− 1 for -SH in addition to the -SO2 functional group. In the mass spectrum of 5h the molecular ion peak was observed at m/z 319 (M+, 2). In the 1H NMR spectrum of 5h, measured in acetone-d6, the following important signals were observed: δ = 1.51 (d, 3H, CH3, J = 7.0 Hz), 4.65–4.73 (m, 1H, CH), 7.23 (bs, 1H, NH, replaceable with D2O), 7.58 (d, 2H, ArH, J = 8.0 Hz), 7.83 (d, 2H, ArH, J = 8.0 Hz) and 12.86 (bs, 1H, NH + SH) with [α´]20D = +42.45o(1.04 g / 100 cm3 of acetone). Spectral, optical and crystal structure data confirmed the presence of only one enantiomer in the case of chiral compounds. Sulfonamides 5a, 5c–e, 5h–j, 6c–d and 6h are new compounds not reported previously in the literature. None of these compounds has been investigated earlier for their antimalarial activities.
Conclusion
A series of new benzenesulfonamides, most of which are chiral, incorporating a 1, 3, 4-oxadiazole and selected amino acid entities have been synthesized using a cost-effective novel approach starting with known reagents and amino acids, via the ester and carbohydrazide intermediate, followed by cyclization with carbon disulfide. Some of these compounds have been investigated for their antimalarial activity. Compound 4h was found to be a potent inhibitor of the degradation of hemoglobin from Plasmodium berghei (ANKA strain). It is worthwhile to note that none of these compounds has been investigated earlier for their antimalarial activities, therefore, they may be considered as very interesting lead molecules for the possible design of novel selective antimalarial drugs.
Acknowledgements
The authors wish to express their sincere thanks to Prof. Jose N. Dominguez, Laboratorio de Bioquimica. Facultad de Farmacia. Universidad Central de Venezuela, for conducting the antimalarial studies.
References
- Shenai BR, Lee BJ, Hernandez AA, Chong PY, Emal CD, Neitz RJ, Roush WR, Rosenthal PJ. Structure-activity relationships for inhibition of cysteine protease activity and development of plasmodium falciparum by peptidyl vinyl sulfones. Antimicrob Agents Chemother 2003; 47: 154–160
- Korolkovas A. Essentials of medicinal chemistry 19882nd ed. John Wiley & Sons, USA 1988; 647–649
- Krungkrai J, Scozzafava A, Reungprapavut S, Krungkrai SR, Rattanajak R, Kamchonwongpaisan S, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of plasmodium falciparum carbonic anhydrase with aromatic sulfonamides towards antimalarials with a novel mechanism of action. Bioorg Med Chem 2005; 13: 483–489
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003; 23: 146–189
- Clark WG, Brater DC, Johnson AR. Medical pharmacology13th ed. Mosby Year Book, USA 1992; 637
- Xia G, Li J, Peng A, Lai S, Zhang S, Shen J, Liu Z, Chen X, Ji R. Synthesis and phosphodiesterase 5 inhibitory activity of novel pyrido[1,2-e] purin-4(3H)-one derivatives. Bioorg Med Chem Lett 2005; 15: 2790–2794
- Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg Med Chem Lett 2004; 14: 217–223
- Casini A, Scozzafava A, Mastrolorenzo A, Supuran CT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets 2002; 2: 55–75
- Rahman A, Farghaly AH. Synthesis reactions and antimicrobial activity of some new indolyl-1,3,4- oxadiazole, triazole and pyrazole derivatives. J Chin Chem Soc 2004; 51: 147–156
- Khanum SA, Shashikanth S, Umesha S, Kavitha R. Synthesis and antimicrobial study of novel heterocyclic compounds from hydroxybenzophenones. Eur J Med Chem 2005; 40: 1156–1162
- Zheng X, Li Z, Wang Y, Chen W, Huang Q, Liu C, Song G. Synthesis and insecticidal activities of novel 2,5- disubstituted 1,3,4- oxadiazoles. J Fluorine Chem 2003; 117: 163–169
- Al-Soud YA, Al-Deeri MN, Al-Mosoudi NA. Synthesis, antitumor and antiviral properties of some 1,2,4- triazole derivatives. Il Farmaco 2004; 59: 775–783
- El-Emam AA, Al-Deeb OA, Al-Omar M Lehmann. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl) -2- substituted thio-1,3,4- oxadiazoles and 5-(1-adamantyl) -3- substituted aminomethyl -1,3,4- oxadiazoles -2-thiones. Bioorg Med Chem 2004; 12: 5107–5113
- Hutt MP, ElLger EF, Werbel LM. 2-Phenyl-5-(trichloromethyl)-1,3,4- oxadiazoles, a new class of antimalarial substances. J Heterocycl Chem 1970; 7: 511–518
- Neamati N, Mazumder A, Sunder S, Owen JM, Schultz RJ, Pommier Y. 2-Mercaptobenzenesulfonamides as a novel class of human immunodeficiency type 1 (HIV-1) integrase and HIV-1 replication. Antimicrob Agents Chemother 1997; 8: 485–495
- Louie AY, Meade TJ. Metal complexes as enzyme inhibitors. Chem Rev 1999; 99: 2711–2734
- Young SD, Britcher SF, Tran LO, Payne LS, Lumma WC, Lyle TA, Huff JR, Anderson PS, Olsen DB, Carrol SS, Pettibone DJ, Obrien JA, Ball RG, Ballani SK, Lin JH, Chen WI, Schleif WA, Sardana VV, Long WJ, Byrnes VW, Emini EA. L- 743,726 (DMP): A novel, highly potent, nonnucleoside inhibitor of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother 1995; 39: 2602–2605
- Brzozowski Z, Saczewski F, Sanchez T, Kuo CL, Gdaniec M, Neamati N. Synthesis, antimicrobial, and anti-HIV-1 integrase activities of 3-aroyl-1,1-dioxo-1,4,2- benzodithiazines. Bioorg Med Chem 2004; 12: 5107–5113
- Lai JYQ, Ferguson Y, Jones M. Preparation of substituted quinolinyl and isoquinolinyl sulfonyl chlorides for synthesis of novel sulfonamides. Synh Commun 2003; 33: 3427–3433
- Furniss BS, Hannaford AJ, Rogers V, Smith PWG, Tatchell AR. Vogel textbook of practical organic chemistry4th ed. Longmann, London 1978, a: pp 1135; b: pp 505 c: pp 1242
- Yousif MY, Ismail AM, Elman AA, El-Kerdawy MM. Synthesis of substituted mercapto-1,2,4-triazoles and substituted 1,2,4-triazoles-5-ylmercaptomethyl-1,3,4-oxadiazoles as antimicrobal agents. J Chem Soc Pak 1986; 8: 183
- Ahmad R, Iqbal R, Akhtar H, Haq ZU, Duddeck H, Stefaniak L, Sitkowski J. Synthesis and structure determination of some oxadiazole-2-thione and triazole-3-thione galactosides. Nucleosides, Nucleotides and Nucleic Acids 2001; 20: 1671–1682
- Baelmans R, Deharo E, Muñoz V, Sauvain M, Ginsburg H. Experimental conditions for testing the inhibitory activity of chloroquine on the formation of beta-hematin. Exp Parasitol 2000; 4: 243–248
- Mitsuya H, Weinhold KJ, Furman PA, St. Clair MH, Lehrmann SN, Gallo RC, Bolognesi D, Barry DW, Border S. 3′-Azido-3′-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci USA 1985; 82: 7096–8000
- Deharo E, Gautret Ph, Ginsburg H, Chabaud AG, Landau I. Synchronization of plasmodium yoelii nigeriensis and P.y. killicki infection in the mouse by means of percoll-glucose gradient stage fractionation: Determination of the duration of the schizogonic cycle. Parasitol Res 1994; 80: 159–164
- Rosenthal P. Plasmodium falciparum: Effects of proteinase inhibitors on globin hydrolysis by cultured malaria parasites. Exp Parasitol 1995; 80: 272–281
- Peters W. Chemotherapy and drug resistance in malaria2nd ed. Academic Press, New York 1987