Abstract
The antioxidant role of novel N-substituted indole-2-carboxamides (I2CDs) was investigated for their inhibitory effects on superoxide anion (O2− ) and lipid peroxidation (LP). Among the synthesized I2CDs, 3, 4, 6, 8 and 9 significantly inhibited O2· − with an inhibition range at 70–98%. Examination of substituent effects on activity showed that both the ortho- and para-positions of the benzamide residue needs to be dichlorinated in order to get a maximum inhibitory effect on superoxide anion. In general, halogenated derivatives were found more active then the non-halogenated ones. However, none of the I2CDs had a significant inhibitory effects on the level of lipid peroxidation; only compounds 7 and 10 moderately decreased LP levels by over 50% at 10− 3 M concentrations.
Introduction
Interest in the application of antioxidants to medical treatment has recently been growing. A lot of evidence has proved the link between the development of human diseases and oxidative stress. Oxidative stress is caused by the increased activity of ROS (Reactive Oxygen Species) generation and decreased antioxidant level in target cells and tissues [Citation1]. The term ROS includes superoxide anion radical (O2· − ), hydroxide radical (HO⋅), hydroperoxide HO2⋅, alkoxide (LO⋅), peroxide (LOO⋅) and non-radical derivatives of oxygen, such as singlet oxygen (1O2), lipid peroxides or H2O2 [Citation2]. High levels of free radicals can cause damage to biomolecules such as lipids, proteins and DNA within cells [Citation3]. The oxidation of these biomolecules may play an important role in the pathogenesis of inflammatory diseases such as atherosclerosis, aging, Alzheimer's disease, Parkinson's disease, stroke, cancer, and AIDS Citation4-9.
Indoles are very common in the body and the diet, and they participate in many biochemical processes. Indoleamines like tryptamine, seratonin, methoxytryptamine, neurohormones (melatonin), phytohormones (indoleacetic acid and indolepropionic acid), indoleamino acids like l-tryptophan and derivatives (N-acetyltryptophan, l-abrine, tryptophan ethyl ester), indolealcohols (tryptophol and indole-3-carbinol), short peptides containing tryptophan, and tetrahydro-beta-carboline (pyridoindole) alkaloids like the pineal gland compound pinoline acted as radical scavengers and antioxidants in an ABTS [2, 2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid)] assay-measuring total antioxidant activity. Their antioxidant capacity were found to be higher then trolox and ascorbic acid (1 mM) [Citation10]. Trolox is a hydrophilic analogue of α-tocopherol, which has stronger activity than α-tocopherol in microsomal membranes (). Among the reported antioxidant indole derivatives in the literature, indolinic nitroxides, which were synthesized via modification of trolox (), have been tested as antioxidants by Antosiewich et al. [Citation11]. It was found that they were very efficient antioxidants, protecting both lipids and proteins from peroxidation. Recent data from our laboratories showed the important antioxidant effects of N-substituted indole esters [Citation12] and amides [Citation13] (). The relationship between inflammatory and free radical action has suggested the implication of the antioxidant capacity of these indole esters and amides, which were previously synthesized and reported as COX-2 enzyme inhibitors [Citation14,Citation15]. The broad spectrum of the observed antioxidant activity of these indole 2- and 3-carboxamides showed that they can scavenge oxygen free radicals, and some of them also inhibited the O2· − produced by the cyclooxygenase. Hence, these results provide direct evidence of the capability of the tested cyclooxygenase inhibitors to scavenge reactive oxygen species [Citation16].
In the framework of our previous studies, we aimed to synthesize novel N-substituted indole-2 carboxamide derivatives in order to identify possible antioxidant activities and also to rationally guide the design of new analaogues. In this study, we report the antioxidant activity and structure-activity relationships of ten novel synthesized I2CDs.
Materials and methods
Materials
Indole-2-carboxylic acid, p-chlorobenzylamine, p-fluorobenzylamine, 2, 4-dichlorobenzylamine were from Aldrich; deutero chloroform, potassium carbonate, p-fluorobenzyl chloride, benzol, dichloromethane, pyridine, hexane, ethyl acetate were from Merck; deutero dimethylsulfoxide, benzyl bromide, 2, 4-difluorobenzylamine were from Acros; methanol, hydrochloric acid, acetic acid, sodium hydroxide, toluene were from Riedel-de Häen; sulfuric acid, sodium hydride, dimethyl formamide, thionyl chloride were from Fluka; ethanol was from Teknik and nitrogen gase was from Kargaz. Cytochrome c, α-tocopherol and thiobarbituric acid (TBA) were purchased from Sigma Chemicals Co.
Chemistry
Melting points were measured with a capillary melting point apparatus (Electrothermal 9100). 1H-NMR spectra were recorded on a Varian Mercury 400 NMR spectrometer for 400 MHz, with Me4Si as internal standard. Chemical shifts (δ) were reported in parts per million (ppm), and signals were expressed as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). Mass spectra were recorded on a Waters ZQ Micromass LC-MS spectrometer with Electrospray Ionization (ESI) method. Infrared (IR) spectra were measured on a Jasco FT/IR-420. Elemental analysis were done on a Leco-932 CHNS-O analyzer. The column chromatography was accomplished on silica gel 60 (Merck).
General procedure for synthesis of N-substituted Indole-2-carboxamide derivatives (1–10)
1-Benzyl and p-fluorobenzyl indole-2 carboxylic acids (0.002 mol) were refluxed in 5 mL benzene (care-carcinogen) with 2.5 mL SOCl2 for 2 h at 80°C. The solvent and SOCl2 were removed by co-evaporation with toluene (3 × 10 mL). The residue was dissolved in 10 mL chloroform and an equivalent amount of pyridine, the corresponding amine derivatives were added and the mixture was stirred at room temperature overnight. The solvent was evaporated to give the crude compounds, which were purified by silicagel column chromatography (hexane: EtOAc = 8:2) and then recrystallized using appropriate solvents.
N, 1-Dibenzyl-1H-indole-2-carboxamide (1)
Recrystallization from ethanol gave pure 0.527 g of compound 1: Yield 77.5%; m.p. 143–145 °C; Rf1 = 0.72 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.71 (dichloromethane); IR (ν) cm− 1; 1638.23 (CO), 1549.52, 3287.02 (N-H), 1451.17 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.59 (d, 2H, J = 6.0, NH-CH2-Ph), 5.86 (s, 2H, CH2-Ph), 6.42 (t, 1H, NH-CH2-Ph), 6.92 (s, 1H, H-a), 7.09 (d, 2H, J = 8.4, H-2, 6), 7.15 (t, 1H, J = 15.2, H-4), 7.20–7.34 (m, 9H, H-b, c, 3, 5, 2′, 3′, 4′, 5′, 6′), 7.38 (d, 1H, J = 8.4, H-d), 7.64 (d, 1H, J = 7.6, H-e); C23H20N2O: 341.13 (M + 1); Calculated: C,80.72%; H,5.94%; N,8.18 (0.1 H2O). Found: C,80.71; H,5.80%; N,8.21%.
1-Benzyl-N-(4-chlorobenzyl)-1H-indole-2-carboxamide (2)
Recrystallization from ethanol gave 0.580 g of pure compound 2: Yield 77.4%; m.p. 174–178 °C; Rf1 = 0.60 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.75 (dichloromethane); IR (ν) cm− 1: 1641.13 (CO), 1541.81, 3295.75 (N-H), 1450.21 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.56 (d, 2H, J = 6.4, NH-CH2-Ph), 5.85 (s, 2H, CH2-Ph), 6.43 (t, 1H, NH-CH2-Ph), 6.93 (s, 1H, H-a), 7.06 (dd, 2H, Jo = 7.2, Jm1 = 1.6, Jm2 = 2.4, H-2, 6), 7.14-7.31 (m, 9H, H-b, c, 3, 4, 5, 2′, 3′, 5′, 6′), 7.39 (d, 1H, J = 8.8, H-d), 7.65 (d, 1H, J = 7.6, H-e); C23H19N2ClO: 375.23 (M + 1); Calculated: C,73.69; H,5.11%; N,7.47%. Found: C,73.28%; H,5.07%; N,7.50%.
1-Benzyl-N-(4-fluorobenzyl)-1H-indole-2-carboxamide (3)
Recrystallization from ethanol gave 0.523 g of pure compound 3: Yield 73.0%; m.p. 160–163 °C; Rf1 = 0.67 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.81 (dichloromethane); IR (ν) cm− 1: 1640.16 (CO), 1538.92, 3305.39 (N-H), 1450.21 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.56 (d, 2H, J = 6.0, NH-CH2-Ph), 5.85 (s, 2H, CH2-Ph), 6.42 (t, 1H, NH-CH2-Ph), 6.92 (s, 1H, H-a), 6.99 (t, 2H, J = 17.2, H-3′, 5′), 7.07 (dd, 2H, Jo = 7.4, Jm1 = 1.6, Jm2 = 2.0, H-2, 6), 7.13–7.30 (m, 7H, H-b, c, 3, 4, 5, 2′, 6′), 7.38 (d, 1H, J = 8.8, H-d), 7.65 (d, 1H, J = 7.6, H-e); C23H19N2FO: 359.20 (M + 1); Calculated: C,77.08%; H,5.43%; N,7.82%. Found: C,77.02%; H,5.21%; N,7.75%.
1-Benzyl-N-(2, 4-dichlorobenzyl)-1H-indole-2-carboxamide (4)
Recrystallization from ethanol gave 0.555 g of pure compound 4: Yield 67.9%; m.p. 166–167 °C; Rf1 = 0.66 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.80 (dichloromethane); IR (ν) cm− 1: 1643.05 (CO), 1542.77, 3267.79 (N-H), 1451.17 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.62 (d, 2H, J = 6.0, NH-CH2-Ph), 5.81 (s, 2H, CH2-Ph), 6.57 (t, 1H, NH-CH2-Ph), 6.95 (s, 1H, H-a), 7.03 (dd, 2H, J = 6.8, Jm1 = 1.6, Jm2 = 2.4, H-2, 6), 7.14–7.30 (m, 7H, H-b, c, 3, 4, 5, 5′, 6′), 7.37 (d, 1H, J = 9.2, H-d), 7.39 (s, 1H, H-3′), 7.66 (d, 1H, J = 8.0, H-e); C23H18N2Cl2O: 409.27 (M + 1); Calculated: C,67.49%; H,4.43%; N,6.84%. Found: C,67.46%; H,4.55%; N,6.76%.
1-Benzyl-N-(2, 4-difluorobenzyl)-1H-indole-2-carboxamide (5)
Recrystallization from ethanol gave 0.586 g of pure compound 5: Yield 77.9%; m.p. 163–168 °C; Rf1 = 0.61 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.72 (dichloromethane); IR (ν) cm− 1: 1641.13 (CO), 1536.99, 3314.07 (N-H), 1450.21 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.59 (d, 2H, J = 6.0, NH-CH2-Ph), 5.82 (s, 2H, CH2-Ph), 6.49 (t, 1H, NH-CH2-Ph), 6.78–6.84 (m, 2H, H-3′, 5′), 6.93 (s, 1H, H-a), 7.05 (d, 2H, J = 8.0, H-2, 6), 7.13–7.30 (m, 6H, H-b, c, 3, 4, 5, 6′), 7.37 (d, 1H, J = 8.0, H-d), 7.65 (d, 1H, J = 7.6, H-e); C23H18N2F2O: 377.17 (M + 1); Calculated: C,73.39%; H,4.82%; N,7.44%. Found: C,73.13%, H,4.77%, N,7.39%.
N-Benzyl-1-(4-fluorobenzyl)-1H-indole-2-carboxamide (6)
Recrystallization from ethanol gave 0.496 g of pure compound 6: Yield 69.2%; m.p. 135–137 °C; Rf1 = 0.61 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.78 (dichloromethane); IR (ν) cm− 1: 1637.27 (CO), 1546.63, 3292.14 (N-H), 1452.14 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.60 (d, 2H, J = 6.0, NH-CH2-Ph), 5.81 (s, 2H, CH2-Ph), 6.45 (t, 1H, NH-CH2-Ph), 6.92 (s, 1H, H-a), 6.93 (t, 2H, J = 17.2, H-3, 5), 7.08 (dd, 2H, Jo = 8.2, Jm1 = 5.6, Jm2 = 5.2, H-2, 6), 7.16 (t, 1H, J = 14.8, H-4′), 7.25–7.34 (m, 6H, H-b, c, 2′, 3′, 5′, 6′), 7.37 (d, 1H, J = 8.4, H-d), 7.65 (d, 1H, J = 7.6, H-e); C23H19N2FO: 359.06 (M + 1); Calculated: C,77.08%; H,5.34%; N,7.82%. Found: C,76.70%, H,5.46%; N,7.62%.
N-(4-Chlorobenzyl)-1-(4-fluorobenzyl)-1H-indole-2-carboxamide (7)
Recrystallization from ethanol gave 0.497 g of pure compound 7: Yield 63.3%; m.p. 161–164 °C; Rf1 = 0.47 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.79 (dichloromethane); IR (ν) cm− 1: 1635.34 (CO), 1541.81, 3298.14 (N-H), 1450.21 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.56 (d, 2H, J = 6.4, NH-CH2-Ph), 5.79 (s, 2H, CH2-Ph), 6.46 (t, 1H, NH-CH2-Ph), 6.92 (t, 2H, J = 17.2, H-3, 5), 6.93 (s, 1H, H-a), 7.06 (dd, 2H, Jo = 8.6, Jm1 = 5.2, Jm2 = 5.6, H-2, 6), 7.17-7.21 (m, 2H, H-2′, 6′), 7.26–7.32 (m, 4H, H-b, c, 3′, 5′), 7.37 (d, 1H, J = 8.4, H-d), 7.65 (d, 1H, J = 8.0, H-e); C23H18N2FClO: 393.14 (M + 1); Calculated: C,70.32%; H,4,62%; N,7,13%. Found: C,70,07%; H,4,61%; N,7,13%.
N, 1-Bis (4-fluorobenzyl)-1H-indole-2-carboxamide (8)
Recrystallization from ethanol gave 0.519 g of pure compound 8: Yield 69.0%; m.p. 142–145 °C; Rf1 = 0.48 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.75 (dichloromethane); IR (ν) cm− 1: 1639.20 (CO), 1550.49, 3298.14 (N-H), 1452.14 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.56 (d, 2H, J = 6.0, NH-CH2-Ph), 5.80 (s, 2H, CH2-Ph), 6.43 (t, 1H, NH-CH2-Ph), 6.92 (s, 1H, H-a), 6.94 (t, 2H, J = 17.2, H-3, 5), 7.01 (t, 2H, J = 17.2, H-3′, 5′), 7.07 (dd, 2H, J = 8.4, Jm1 = 4.8, Jm2 = 5.6, H-2, 6), 7.16 (t, 1H, J = 16.0, H-c), 7.22 (dd, 2H, J = 8.4, Jm1 = 5.6, Jm2 = 5.6, H-2′, 6′), 7.29 (t, 1H, J = 16.0, H-b), 7.37 (d, 1H, J = 8.4, H-d), 7.65 (d, 1H, J = 8.0, H-e); C23H18N2F2O: 377.14 (M + 1); Calculated: C,71.67%; H,4.96%; N,7.26% (0.5 H2O). Found: C,71.47%; H,4.87%; N,7.32%.
N-(2, 4-Dichlorobenzyl)-1-(4-fluorobenzyl)-1H-indole-2-carboxamide (9)
Recrystallization from ethanol gave pure 0.636 g of compound 9: Yield 74.5%; m.p. 157–159 °C; Rf1 = 0.58 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.85 (dichloromethane); IR (ν) cm− 1: 1638.23 (CO), 1541.81, 3283.21 (N-H), 1450.21 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.62 (d, 2H, J = 6.4, NH-CH2-Ph), 5.76 (s, 2H, CH2-Ph), 6.61 (t, 1H, NH-CH2-Ph), 6.89 (t, 2H, J = 17.6, H-3, 5), 6.95 (s, 1H, H-a), 7.03 (dd, 2H, J = 8.8, Jm1 = 4.8, Jm2 = 5.6, H-2, 6), 7.15-7.28 (m, 4H, H-b, c, 5′, 6′), 7.36 (d, 1H, J = 8.4, H-d), 7.40 (d, 1H, Jm = 2.0, H-3′), 7.66 (d, 1H, J = 8.0, H-e); C23H17N2FCl2O: 427.12 (M + 1); Calculated: C,64.37%; H,4.04%; N,6.52% (0.1 H2O). Found: C,64.20%; H,4.05%; N,6.53%.
N-(2, 4-Difluorobenzyl)-1-(4-fluorobenzyl)-1H-indole-2-carboxamide (10)
Recrystallization from ethanol gave 0.482 g of pure compound 10: Yield 61.1%; m.p. 127–129 °C; Rf1 = 0.59 (n-hexane:ethyl acetate; 7:3), Rf2 = 0.81 (dichloromethane); IR (ν) cm− 1: 1637.27 (CO), 1544.70, 3289.00 (N-H), 1451.17 (C-N); H1-NMR (400 MHz, CDCl3) δ 4.59 (d, 2H, J = 6.0, NH-CH2-Ph), 5.77 (s, 2H, CH2-Ph), 6.51 (t, 1H, NH-CH2-Ph), 6.83 (t, 2H, J = 16.8, H-3′, 5′), 6.90 (t, 2H, J = 17.6, H-3, 5), 6.93 (s, 1H, H-a), 7.05 (dd, 2H, J = 8.6, Jm1 = 5.6, Jm2 = 5.2, H-2, 6), 7.16 (t, 1H, J = 15.6, H-c), 7.24-7.31 (m, 2H, H-b, 6′), 7.35 (d, 1H, J = 8.0, H-d), 7.66 (d, 1H, J = 8.0, H-e); C23H17N2F3O: 395.10 (M + 1); Calculated: C,70.04%; H,4.34%; N,7.10%. Found: C,69.90%; H,4.61%; N,6.86%.
Antioxidant properties of novel Indole derivatives
Superoxide radical scavenging activity
The capacity of I2CDs that scavenge superoxide anion formation (SOD) was determined spectrophotometrically on the basis of inhibition of cytochrome c reduction following the modified method of McCord et al. [Citation17]. Superoxide anion was generated in the xanthine/xanthine oxidase system. The reaction mixture contained in a final volume of 1.0 mL, 0.05 M phosphate buffer pH 7.8, 0.32 Units/mL xanthine oxidase, 50 μM xanthine, 60 mM ctytochrome c and different concentration of synthesized I2CDs at 100 μL. The reaction was started by the addition of xanthine oxidase. The absorbance was measured spectrophotometrically at 550 nm for cytochrome c reduction. Each test was performed in 2–4 experiments, and the result was expressed as a percent of the control.
Assay of lipid peroxidation
The effect of synthesized compounds on rat liver homogenate lipid peroxidation level in the presence of FeCl2-ascorbic acid was determined by the modified method of Mihara et al. [Citation18]. Male albino Wistar rats (200–225 g) were fed a standard laboratory rat chow and allowed to drink tap water ad libitum. Procedures involving the animals and their care conformed to Institutional guidelines, in compliance with National and International laws and guidelines for the use of animals in biomedical research. The animals were starved for 24 h prior to execution by decapitation under anesthesia. The livers were immediately removed and washed in ice-cold distilled water, and then immediately homogenized with an ice-chilled Teflon homogenizer. LP was measured spectrophotometrically by estimation of thiobarbituric acid reactant substances (TBARS). Amounts of TBARS were expressed in terms of nmol malondialdehyde (MDA)/g tissue. This optimized assay mixture contained 0.5 mL of liver homogenate, 0.1 mL of Tris-HCl buffer (pH 7.2), 0.05 mL of 0.1 mM ascorbic acid, 0.05 mL of 4 mM FeCl2 and 0.05 mL of various concentrations of the synthesized compounds, or α-tocopherol (Vit E). The mixture was incubated for 1 h at 37°C. After incubation, 3.0 mL of H3PO4 and 1.0 mL of 0.6% TBA were added and the mixture was shaken vigorously. The mixture was then boiled for 30 min, cooled and n-butanol added. The whole mixture was then again shaken vigorously. The n-butanol phase was separated by centrifugation at 3000 rpm for 10 min. The absorbance of the supernatant was measured at 532 nm against a blank, which contained all reagents except liver homogenate.
Results and discussion
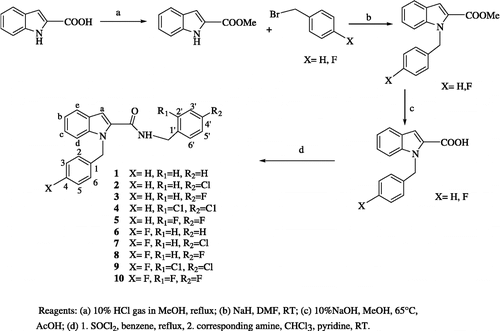
The target I2CDs were synthesized as described in Scheme . Briefly, the indole-2-carboxylic acid was reacted with thionyl chloride and the resulting crude acid chloride was transformed into the corresponding benzamides [Citation19,Citation20].
NMR, IR, Mass spectrometry and elemental analysis confirmed the structure of all the compounds. In the IR spectra, absorption bands for all compounds were detected in the range 1638–1643 cm− 1 corresponding to a CO stretching vibration. NH stretching and bending values were found as 3283–3305 cm− 1 and 1536–1550 cm− 1 respectively. The C-N stretching vibration of the compounds occurred in the range 1450–1452 cm− 1. In the NMR the characteristic NH triplets of CONH-CH2-Ph were found at 6.42–6.57 ppm and the CH2 protons were found as doublets at 4.56–4.62 ppm with a coupling constant 6.0–6.4 Hz. N-Substituted benzyl protons were observed as a sharp singlet at 5.76–5.86 ppm. The chemical shifts of all aromatic protons were detected at 6.81–7.65 ppm. The characteristic indole protons were as follows: 6.91–6.93 ppm (indole a protons), 7.03–7.33 ppm (indole b and c protons), 7.35–7.39 ppm (indole d protons) and 7.64–7.66 ppm (indole e protons).
The antioxidative properties of the synthesized I2CDs were studied by determining their inhibitory effect on superoxide anion (O2· − ) and lipid peroxidation. The activity results were compared with α-tocopherol and are presented in ; a comparable graphical analysis is given in . The scavenging extent of compounds 3, 4, 6, 8 and 9 were in the range of 70–100% at 10− 3 M concentration. Vit E caused 83% inhibition on superoxide anion production at the same concentration. The compounds were also tested for inhibition of superoxide anion formation at a concentration of 10− 4 M. Compounds 4 and 9 had scavenging effects on the superoxide anion radical (22% and 19%, respectively). Among all the compounds, 1, 3, 5, 6, 7, 8, and 10 decreased the level of LP by 41–67% at 10− 3 M concentration. As seen in , only compound 10 almost equally inhibited the LP level by 67% at 10− 3 M and by 70% at 10− 4 M concentrations, respectively.
Table I. Inhibitory effects of compounds 1–10 on superoxide anion production and LP.
Figure 2 Percentage inhibition by compounds of liver LP level and liver superoxide anion production at 10− 3M concentration.
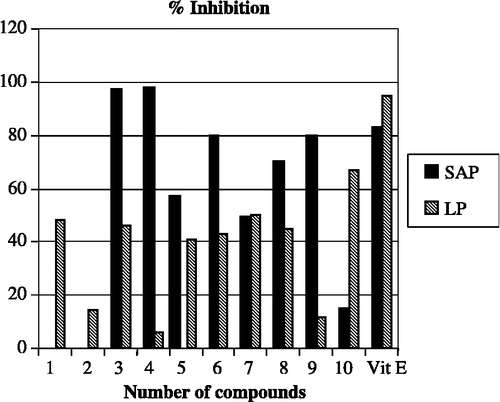
When we examine the effects of substituents on activity, it was found that dichloro-substitution of the benzamide ring (compounds 4 and 9), led to 80–98% inhibition. The introduction of a halo substituent, i.e. fluoro and chloro, in the para-position of the aromatic ring of benzamide did not significantly alter the activity (compounds 2 and 7). This suggested that both ortho- and para-positions of the aromatic ring of benzamide should be dichlorinated in order to get maximum inhibitory effects on the superoxide anion. On the other hand, the presence of a para-fluorine atom (compounds 3 and 8) in the aromatic ring of benzamide showed more a positive effect on activity than the ortho-, para- difluoro substituted compounds (5 and 10). Comparison of the activity results of halogenated and non-halogenated derivatives revealed that halogenated compounds are generally more active than the non-halogenated ones. However, the non-halogenated benzamide compound 6 expressed 80% superoxide anion scavenging activity. The introduction of a para-fluoro benzyl ring on the indole nitrogen is believed to have a positive effect for the inhibitory effect of this compound. On the other hand, the presence of a para-fluorobenzyl substituent at position-1 of the indole moiety was not beneficial for the inhibition of superoxide anion for other compounds. However, further investigation is required to clarify this issue.
None of the I2CDs have significant inhibitory effects on the level of lipid peroxidation in comparison with Vit E. This difference is not surprising as the mechanism of production of oxidative stress (or reactive oxygen species) in these assays (SOD and LP inhibition) are different [Citation21]. The structure of biological membranes is very heterogenous; each membrane is built up of particular lipids and proteins. For instance, antioxidants that protect lipids do not necessarily protect proteins from peroxidation, as these two processes can occur independently [Citation22]. Furthermore, if any particular antioxidant structure allows protection of the specific proteins, this does not imply that that particular antioxidant will also protect all proteins from peroxidation. The superoxide anion radical has been implicated in several pathophysiological processes, due to its transformation into more reactive species including hydroxyl radical that initiates lipid peroxidation [Citation23]. Therefore, it can still be concluded that, the scavenging of superoxide anion radical by 3, 4, 6, 8, 9 (70–98%, at 10− 3 M concentration) is likely to make them promising antioxidants. Further studies are to be conducted to investigate the relationship between different type of substituents and their positions in the I2CDs on antioxidant activity especially on superoxide anion inhibition.
In our previous studies, we have reported that the occurence of carbon-centered radicals could be responsible for the possible antioxidant mechanism of indole amide derivatives [Citation13]. The results suggested that the antioxidant properties of the amides had a direct effect on the scavenging of hydroxyl radicals. Since LP experiment from this study did not result in significant activity, it could be considered that abstraction of an electron from the indole ring by hydroxyl radical cannot initiate reaction.
Acknowledgements
The authors thank Prof. Dr Hakan Göker for providing NMR and Mass spectral analysis and also special thanks to M.Sc Mehmet Alp for Elemental analysis.
References
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47–95
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stres-induced cancer. Chem-Biol Inter 2006; 160: 1–40
- Thompson HJ. DNA oxidation products, antioxidant status and cancer prevention. J Nutr 2004; 134: 3186S–3187S
- Valko M, Mario I, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 2004; 266: 37–56
- Ames NB, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative disease of aging. Proc Natl Acad Sci USA 1993; 90: 7915–7922
- Bendheim PE, Poeggeler B, Neria E, Ziv V, Pappolla M, Chain DG. Development of indole-3-propionic acid (Oxigon) for Alzheimer's diseses. J Mol Nurosci 2002; 19: 213–217
- Andreadou I, Tsantili-Kakaolidou A, Spyropoulou E, Siatra T. Reactions of indole derivatives with cardioprotective activity with reactive oxygen species. Comparison with melatonin. Chem Pharm Bull 2003; 51(10)1128–1131
- Collins AR. Antioxidant intervention as a route to cancer prevention. Eur J Cancer 2005; 41: 1923–1930
- Seo JY, Kim HY, Seo JT, Kim KH. Oxidative stress induced cytokine production in isolated pancreatic acinar cells: Effects of small-molecule antioxidants. Pharmacology 2002; 64(2)63–70
- Herraiz T, Galisteo J. Endogenous and dietary indoles: A class of antioxidants and radical scavenging in the ABTS assay. Free Rad Res 2004; 38: 323–331
- Antosiowich J, Damiani E, Jassem W, Wozniak M, Orena M, Greci L. Influence of structure on the antioxidant activity of indolinic nitroxide radicals. Free Rad Biol Med 1997; 22(1)249–255
- Olgen S, Coban T. Antioxidant evaluations of novel N-H and N-substituted indole esters. Biol Pharm Bull 2003; 26(5)736–738
- Ölgen S, Çoban T. Synthesis and antioxidant properties of novel N-substituted indole 2-carboxamide and 3-acetamide derivatives. Arch Pharm 2002; 335(7)331–338
- Ölgen S, Nebioğlu D. Synthesis and biological evaluation of N-substituted indole esters as inhibitors of cyclooxygenase 2 (COX-2). Farmaco 2002; 57(8)677–683
- Olgen S, Guner E, Fabregat MA, Crespo MI, Nebioglu D. Syntheses and biological evaluation of indole-2 and 3-carboxamides: A new selective cyclooxygenase-2 inhibitors. Pharmazie 2002; 57(4)238–242
- Aboul-Enein HY, Kladna A, Kruk I, Lichszteld K, Michalska T, Olgen S. Scavenging of reactive oxygen species by N-substituted indole-2- and 3-carboxamides. Luminescence 2004; 19: 1–7
- McCord JM, Fridovich JM. Preparation and assay of superoxide dismutases. Meth Enzymol 1978; 53: 382–393
- Mihara M, Uchiyama MS, Fukuzawa K. Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem Med 1980; 23: 303–311
- Murakami Y, Watanabe T, Kobayashi A, Yokoyama Y. A Novel method for the debenzylation of protected indole nitrogen. Synthesis 1984; 4: 738–740
- Battaglia S, Boldrini E, Da Settimo F, Dondio G, La Motta C, Marini AM, Primofiore G. Indole amide derivatives: Synthesis, structure-activity relationships and molecular modelling studies of a new series of histamine H1-receptor antagonists. Eur J Med Chem 1999; 34: 93–105
- Dix TA, Aikens J. Mechanism and biological relevance of lipid peroxidation initiation. Chem Res Toxicol 1993; 6: 2–18
- Neuzil J, Gebicki JM, Stocker R. Radical-induced chain oxidation of proteins and its inhibition by chain-breaking antioxidants. Biochem J 1993; 293: 601–606
- Ates-Alagoz Z, Kus C, Coban T. Synthesis and antioxidant properties of novel benzimidazoles containing substituted indole or 1, 1, 4, 4-tetramethyl-1, 2, 3, 4-tetrahydro-naphthalene fragments. J Enz Inhib Med Chem 2005; 20(4)325–331