Abstract
An alcoholic extract obtained from the rhizomes of Gloriosa superba Linn (Colchicaceae) was screened for enzyme inhibition activities. The crude extract and its subsequent fractions including chloroform, ethyl acetate, n-butanol and aqueous were screened against lipoxygenase, actylcholinesterase, butyrylcholinesterase and urease. An outstanding inhibition on lipoxygenase was observed. The highest enzyme inhibition potency was expressed by the chloroform fraction (90%) among the tested fractions on lipoxygenase. Overall 67– 90% inhibition was found for lipoxygenase, 46-69% for acetylcholinesterase and 10–33% for butyrylcholinesterase, while urease was not inhibited.
Introduction
Lipoxygenases (LOXs) are non-heme iron containing enzymes ubiquitous in nature. Dioxygenases [cyclooxygenases (COX-1, COX-2) and lipoxygenases (5-LOX, 8-LOX, 12-LOX, and 15-LOX)] are responsible for the metabolism of fatty acids (FA) and their metabolites cause inflammatory responses in the body. They also play a significant role in cancer cell growth, metastasis, invasiveness, cell survival and induction of tumor necrosis factor (TNF) [Citation1,Citation2]. The 12/15-lipoxygenase has a role in inflammatory diseases including atherosclerosis, cancer, osteoporosis, angiotension II-dependent hypertension and diabetes [Citation3]. It has been pointed out, that dietary FA enhances cytotoxicity of some neoplastic agents and the anticancer effects of radiotherapy [Citation4]. The involvement of LOXs in cancer has been well documented and summons our attention to all types of LOXs and many of their products. Inhibition of the 5-LOX pathway has a chemopreventive effect in animal lung carcinogenesis, and blocks the oxidation of several potent carcinogens [Citation5,Citation6]. Inhibitors of 5-LOX have the ability to decrease cell proliferation and trigger apoptosis [Citation7]. Therefore, designing new agents to modulate the activities of the variety of lipoxygenases (LOXs) is timely.
Acetylcholine (ACh) plays a well-recognized role in nerve excitation and is found in cholinergic synapses that provide a stimulatory transmission in the nervous system Citation8-10. Acetylcholinesterase (AChE) hydrolyzes acetylcholine and thereby increasing the concentration of endogenous acetylcholine in the vicinity of cholinoreceptors [Citation11]. Acetylcholinesterase inhibitors are considered effective treatments for cognitive decline in Alzheimer's diseases where the loss of cholinergic neurons is thought to be responsible for various cognitive deficits [Citation12]. Treatment with acetylcholinesterase inhibitors (AChEI) has shown a positive effect on cognitive functions of patients with Alzheimer's disease and other conditions such as Lewy Body dementia, subcortical vascular dementia and Parkinson's disease [Citation13]. Therefore, the enzyme acetylcholinesterase (AChE) has long been an attractive target for rational drug design in the discovery of inhibitors.
The plant Gloriosa superba Linn, commonly known as Climbing lily (English) and Samp ki Bothi (Urdu), belongs to the Colchicaceae family. It is a perennial herb, semi-woody herbaceous, branching climber, reaching approximately 5 metres in height. 1 to 4 stems arise from a single V-shaped fleshy cylindrical tuber. The perianth segments, which are accrescent during anthesis and become reflexed, are striking in color, yellow proximally and at the margins and dark red in the median portion [Citation14,Citation15]. A native of tropical Africa it is now found growing naturally throughout much of tropical Asia including India, Sri Lanka, Malaysia and Burma [Citation16].
In the indigenes system of treatment, different parts of this plant are widely used in tropical Africa and Asia. The important medicinal use is as a source of colchicine, both in the seed and tuberous rhizome, for the treatment of gout [Citation17,Citation18]. The juice of the leaves is used to kill head lice and also as an ingredient in arrow poisons, and has also exhibited mosquito cytogenetic properties [Citation19]. A paste of the root formed with water is found to be effective in the treatment of wounds [Citation20,Citation21]. The tuber also have antidotal properties for snakebite and in India it is commonly placed on windowsills to deter snakes. Many cultures believe the species to have various magical properties Citation22-24. Phytochemically, in addition to colchicine and gloriosine, Gloriosa superba also contains other alkaloid such as 3-desmethyl colchicine, β-lumicolchicine, N-formyldesacetyl-colchicine, 2-desmethyl colchicine, and compounds such as chelidonic acid and salicylic acid [Citation25].
Materials and methods
Plant material
Gloriosa superba Linn, as a whole plant was collected from Swat, N.W.F.P (Pakistan) during the months of July-Aug 2005.The plant material was identified by Mr. Sher Aman, Research Officer Department of Botany, University of Peshawar, and verified by Professor Dr Abd-ur-Rashid, Department of Botany, University of Peshawar.
Extraction
The shade dried plant material was chopped into small pieces and pulverized into a fine powder. The powdered plant material (11.5 Kg) was soaked in methanol with occasional shaking, at room temperature. After 15 days, the methanol soluble materials were filtered off. The filtrate was concentrated under vacuum at low temperature (40°C) using a rotary evaporator. A crude extract (269 g) was obtained.
Fractionation
The crude methanolic extract (269 g) was suspended in distilled water (500 mL) and partitioned with n-hexane (3 × 500 mL), chloroform (3 × 500 mL), ethyl acetate (3 × 500 mL) and n-butanol (3 × 500 mL) to yield the n- hexane (24 g), chloroform (74 g), ethyl acetate (35 g), n-butanol (42 g) and aqueous (61 g) fractions, respectively. The enzyme inhibition activity assays were performed using different concentrations of the crude extract and various fractions as per the requirement of the individual assay method.
Enzyme assays
a) Lipoxygenase inhibition
Lipoxygenase inhibiting activity was conveniently measured by slightly modifying the spectrometric method developed previously [Citation26]. Lipoxygenase 1.13.11.12) type I-B and linoleic acid were purchased from Sigma (St. Louis, MO) and used without further purification. All other chemicals were of analytical grade. 160 mL of sodium phosphate buffer, 0.1 mM (pH 7.0), 10 mL of the sample solution and 20 mL of lipoxygenase solution were mixed and incubated for 5 min at 25.8°C. The reaction was initiated by the addition of 10 uL linoleic acid solution substrate and the absorption change due to the formation of (9Z, 11E, 13S)-13-hydroperoxyoctadeca-9, 11-dienoate was followed for 10 min. The test sample and the control were dissolved in 50% ethanol. All the reactions were performed in triplicate. The IC50 values (concentrations of sample causing 50% reduction in activity relative to the control) were calculated using the EZ-Fit Enzymes kinetics programme.
b) Cholinesterase inhibition
Acetylcholinesterase and butyrylcholinesterase inhibiting activities were measured by slightly modifying the spectrophotometric method previously developed [Citation27]. Electric-eel AChE (type VI-S, Sigma) and horseserum BChE (Sigma) were used as source of the cholinesterases and acetylthiocholine iodide and butyrylthiocholine chloride (Sigma), respectively, were used as substrates in the reaction. 5,5 – Dithiobis (2-nitrobenzoic acid) (DTNB, Sigma) was used for the measurement of cholinesterase activity. 140 mL of sodium phosphate buffer 100 mM, (pH 8.0), 10mL of DTNB, 20 mL of the test sample solutions and 20mL of acetylcholinesterase/ butyrylcholinesterase solution were mixed and incubated for 15 min at 25.8°C. The reactions were then initiated by the addition of l0 mL acetylthiocholine/butyrylthiocholine, respectively. The hydrolysis of acetylthiocholine and butyrylthiocholine was monitored at 412 nm by the formation of the yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released during enzymatic hydrolysis. The samples and the control were dissolved in 50% ethanol. All the reactions were performed in triplicate.
Results and discussion
Terrestrial plants offer a unique and renewable resource in order to discover potential new drugs and biological entities, because of the structural and biological diversity of their constituents. At the same time, global interest is increasing in natural products because of safety concerns for synthetic products [Citation29,Citation30]. In industrialized nations at the present time, some fifty percent of all prescribed drugs are derived or synthesized from natural products. The WHO has estimated that for some 3.4 billion people in the developing world, plants represent the primary source of medicine. Therefore, the current study was designed to investigate and to provide a scientific basis for the use of Gloriosa superba as a herbal remedy. For this purpose invitro enzyme inhibition activities of the crude methanolic extract and subsequent fractions of Gloriosa superba Linn were conducted against acetylcholinesterase, butyrylcholinesterase, lipoxygenase and urease.
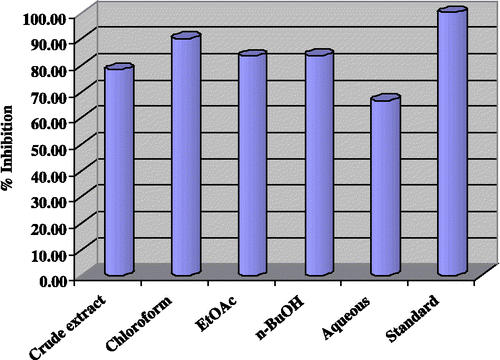
The enzyme inhibition activities towards lipoxygenase obtained in the current study () were highly promising. The highest inhibition (90.10%) was expressed by the chloroform fraction in the assay. A similar trend was displayed by the ethyl acetate fraction (83.50%), n-butanol fraction (83.70%) and crude methanolic extract (78.30%). On the other hand, the aqueous fraction exhibited the least enzyme inhibitory activity (66.70%).
Figure 1 Lipoxygenase inhibition by crude extract and fractions of Gloriosa superba Linn at 240μg/200μL.
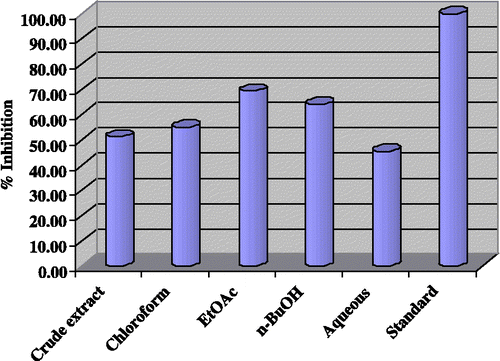
The results obtained with the crude extract and subsequent fractions of Gloriosa superba Linn, for inhibitory activities against acetylcholinesterase are shown in . The ethyl acetate fraction gave the highest inhibition (69.40%), followed by the n-butanol fraction (64.10%) and chloroform fraction (55.10%). The crude methanolic extract gave 51.20% inhibition, while the aqueous fraction demonstrated least inhibition (45.50%).
Figure 2 Acetylcholinesterase inhibition by crude extract and fractions of Gloriosa superba Linn at 40μg/200μL.
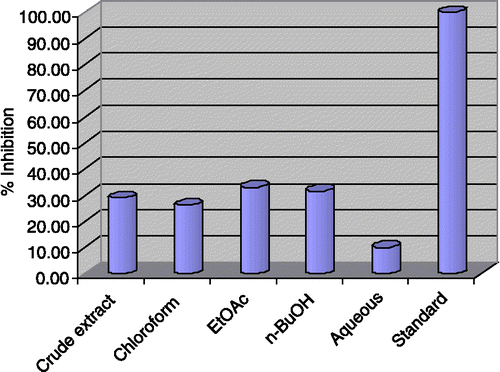
Similarly represents the enzyme inhibition results found against butyrylcholinesterase. Overall, low inhibitory potency was displayed by the crude extract and subsequent fractions of Gloriosa superba Linn against butyrylcholinesterase; the crude extract exhibited 29.10% inhibition, chloroform fraction 26.30%, ethyl acetate fraction 33.10%, n-butanol fraction 31.70% and the aqueous fraction 10%. However, no enzyme inhibitory activity was found against urease.
Figure 3 Butyrylcholinesterase inhibition by the crude extract and fractions of Gloriosa superba Linn at 40 μg /200 μL.
The crude extract and subsequent fraction of the rhizomes of Gloriosa superba Linn expressed an outstanding inhibitory activity on lipoxygenase. Therefore, it can be assumed that this plant species could be an excellent source of natural lipoxygenase inhibitor for various therapeutic purposes. Although, the phytochemical studies of the plant has confirmed the presence of colchicine type alkaloids that may be contributory to this inhibitory activity, comprehensive investigations are required in order to explore the exact mechanism of this activity, and confirm other uses of the plant in the indigenous system of medicine.
References
- Chan MM. Inhibition of tumor necrosis factor by curcumin, phytochemical. Biochem Pharmacol 1995; 49: 1551–1556
- Hallahan DE, Virudachalam S, Kufe DW, Weichselbaum RR. Ketoconazole attenuates radiation-induction of tumor necrosis factor. Int J Rad Oncol Biol Phys 1994; 29: 777–780
- Hartmut Kühn, O'Donnell Valerie B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res 2006; 45: 334–356
- Conklin KA. Dietary polyunsaturated fatty acids: impact on cancer chemotherapy and radiation. Altern Med Rev 2002; 7: 4–12
- Rioux N, Castonguay A. Inhibitors of lipoxygenase: A new class of cancer chemopreventive agents. Carcinogenesis 1998; 19: 1393–1400
- Moody TW, Leyton J, Martinez A, Hong S, Malkinson A, Mulshine JL. Lipoxygenase inhibitors prevent lung carcinogenesis and inhibit non-small cell lung cancer growth. Exp Lung Res 1998; 24: 617–628
- Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci USA 1998; 95: 13182–13187
- Llinás RR. The squid giant synapse: A model for chemical transmission. Oxford University Press, London 1999
- Paez MC, Fayad R. Diffusion of the neurotransmitter cannot govern the rise time of miniature end plate. Revista Colombiana de Física 1999; 31: 163
- Guyton CA, Hall JE. Textbook of medical physiology (tenth ed.). W.B. Saunders Company, Amsterdam 2000
- Bertram G, Katzung. Basic and clinical pharmacology9th Ed. 2004; 102
- Tonmoy Sharma, Catherine Reed, Ingrid Aasen, Veena Kumari. Schizophrenia Res 2006; 85: 73–83
- Porcel J, Montalban X. Anticholinesterasics in the treatment of cognitive impairment in multiple sclerosis. J Neurolog Sci 2006; 245: 177–181
- Smith AC. Flora Vitiensis nova: A new flora of Fiji. Lawai, Kauai, Hawaii. National Tropical Botanical Garden 1979; 1: 141–142
- Huxley A, ed-in-chief. The Royal Horticultural Society dictionary of gardening. MacMillan Press, London 1992; Vol 2
- Jayaweera DMA. Medicinal plants used in Ceylon. National Science Council of Sri Lanka (part 3), Colombo 1982
- Anon. The wealth of india: A dictionary of raw material and industrial products. CSIR, Delhi 1956; Vol. 4: 139–140
- Bhakuni DS, Jain Sudha. Advances in horticulture, KL Chadha, Rajendra Gupta. Malhotra Publishing House, Delhi 1995; 11: 98–99
- Choochote W, Rongsriyam K, Pitasawat B, Jitpakdi A, Rattanachanpichai E, Junkum A, Tuetun B, Chaiwong P. Evaluation of the colchicine-like activity of gloriosa superba-extracted fractions for mosquito (Diptera: Culicidae) cytogenetic study. J Med Entomol 2004; 41: 672–676
- Biswas TK, Mukherjee B. Plant medicines of Indian origin for wound healing activity: A review. Intern J Lower Extremity Wounds 2003; 2: 25–39
- Katewa SS, Chaudhary BL, Jain A. Folk herbal medicines from tribal area of Rajasthan, India. J Ethnopharmacol 2004; 92: 41–46
- Watt JM, Breyer-Brandwijk MG. The medicinal and poisonous plants of southern and eastern africa. E. & S. Livingstone, Edinburgh 1962
- Neuwinger HD. African ethnobotany. Poisons and drugs. Chemistry, pharmacology, toxicology. Chapman & Hall, Weinheim 1994, English translation by A Porter
- Burkill HM. The useful plants of West Tropical Africa2nd ed. Royal Botanic Gardens, Kew 1995; Vol. 3
- Duke JA. Handbook of medicinal herbs. CRC Press, USA 1985
- Tappel AL. Methods in enzymology. Academic Press 1962; 5: 539–542
- Ellman GL, Courtney KD, V. Jr, Andres RM. Featherstone, A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88–95
- Weatherburn MW. Phenol-hypochlorite reaction for the determination of the ammonia. Anal Chem 1967; 39: 971–974
- Gijtenbeek JMM, Vanden Bent MJ, Vecht CJ. Cyclosporine neurotoxicity. J Neurol 1999; 246: 339–346
- Johnson WC, William OW. Warfarin toxicity. J Vasc Surg 2002; 35: 413–421
