Abstract
The increasing clinical importance of drug-resistant mycobacterial pathogens has lent additional urgency to microbiological research and new antimycobacterial compound development. For this purpose, new triazoles were synthesized and evaluated for antituberculosis activity. A series of 4-arylidenamino-4H-1,2,4-triazole-3-thiol derivatives (2a–n) were synthesized from the treatment of 4-amino-4H-1,2,4-triazoles-3-thiol (1) with the respective aldehydes and were evaluated for antituberculosis activity against Mycobacterium tuberculosis H37Rv (ATCC 27294), using the BACTEC 460 radiometric system and BACTEC 12B medium. Compound 2k showed an intereting activity at 6.25 μg/mL with a 87 percentage inhibition.
Introduction
Humankind's battle with tuberculosis (TB) dates back to antiquity. TB, which is caused by Mycobacterium tuberculosis, was a much more prevalent disease in the past than it is today, and it was responsible for the deaths of about one billion people during the last two centuries [Citation1]. TB is on the increase in recent years, largely owing to HIV infection, immigration, increased trade, and globalization [Citation2]. The increasing emergence of drug-resistant TB, especially multidrug-resistant TB (MDR-TB, resistant to at least two frontline drugs such as isoniazid and rifampicin), is particularly alarming and standard TB therapy is ineffective in controlling MDR-TB in high MDR-TB incidence areas Citation3-4. There is much concern that the TB situation may become even worse with the spread of HIV worldwide, a virus that weakens the host immune system and allows latent TB to reactivate so making the person more susceptible to re-infection with either drug-susceptible or drug resistant strains. The lethal combination of drug-resistant TB and HIV infection is a growing problem that presents serious challenges for effective TB control. In view of this situation, the World Health Organization (WHO) in 1993 declared TB a global emergency [Citation5].
There is an urgent need to develop new TB drugs [Citation6]. However, no new TB drugs have been developed over the last 40 years. Although TB can be cured with the current therapy, the six months needed to treat the disease is too long, and the treatment often has significant toxicity. These factors make patient compliance to therapy very difficult, and this noncompliance frequently selects for drug-resistant TB bacteria. The current TB problem clearly demonstrates the need for a re-evaluation of our knowledge of the current TB drugs and chemotherapy and the need for new and better drugs that are not only active against drug-resistant TB but also, more importantly, shorten the requirement for six months of therapy [Citation7].
Earlier studies in our laboratory have identified various 1,2,4-triazole derivatives exhibiting chemotherapeutic properties against Mycobacterium tuberculosis [Citation8]. As a continuation of our efforts to develope broad-spectrum chemotherapeutics, we undertook the present study to design, synthesize and evaluate 1,2,4-triazole analogues, as potential inhibitors of the opportunistic Mycobacterium tuberculosis microorganism.
Experimental
Chemistry
All reagents were used as purchased from commercial suppliers without further purification. Melting points were determined by using an Electrothermal 9100 digital melting point apparatus and were uncorrected. The compounds were checked for purity by TLC on silica gel 60 F254 (Merck). Elemental analyses were performed on a Perkin Elmer EAL 240 elemental analyser. Spectroscopic data were recorded on the following instruments: IR (KBr disc, v, cm− 1) on a Shimadzu 435 IR spectrophotometer; 1H-NMR spectra (δ, ppm, Hz) were recorded on a Bruker spectrometer (250 MHz) in DMSO-d6 with TMS as an internal standard; MS-FAB+, on a VG Quattro mass spectrometer.
General procedure for synthesis of the compounds
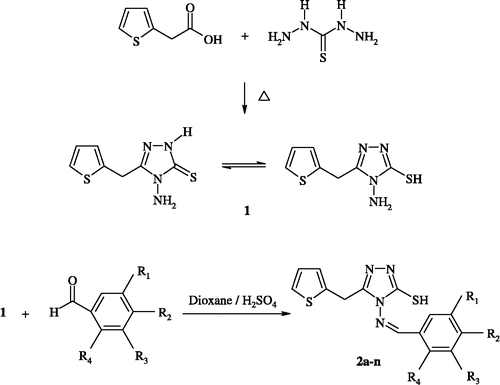
4-Amino-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (1)
A mixture of thiocarbohydrazide (0.1 mol) and thiophene-2-acetic acid (0.1 mol) was heated in an oil bath at 160–170°C for 2 h. The fused mass thus obtained was dispersed with hot water to obtain the triazole. The product was recrystallized from methanol [Citation8].
4-arylidenamino-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol derivatives (2a–n)
To a suspension of arylaldehyde (5 mmol) in dioxane (10 ml), was added an equimolar amount of triazole (1). The suspension was heated until a clear solution was obtained. A few drops of conc. sulfuric acid were added as a catalyst and the solution was refluxed for 3 h on a water bath. The precipitated solid was filtered off and recrystallized from ethanol.
Some characteristics of the synthesized compounds are shown in . Analytical and spectral data (IR, 1H-NMR, FAB+-MS) confirmed the structures of the new compounds.
Table I. Some characteristics of compounds (2a–n).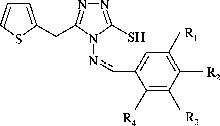
4-(benzylideneamino)-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2a):
IR [ν, cm− 1, KBr]: 3328 (N–H), 2568 (SH), 1630 (C = N), 1545, 1262, 1052, 950 (N–C = S, amide I, II, III and IV bands), 723 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 4.35 (2H, s, CH2), 6.91 − 7.93 (8H, m, aromatic protons), 10.05 (1H, s, –N = CH), 13.85 (1H, brs, SH). MS-FAB+: m/z: 300 (M+), 301 (M++1). For C14H12N4S2 calculated: 55.98 C, 4.03 H, 18.65 N; found: 56.11 C, 4.18 H, 18.71% N.
4-[(4-fluorobenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2b):
IR [(ν, cm− 1, KBr]: 3330 (N–H), 2525 (SH), 1633 (C = N), 1535, 1260, 1050, 954 (N–C = S, amide I, II, III and IV bands), 715 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 4.39 (2H, s, CH2), 6.94–8.10 (7H, m, aromatic protons), 10.10 (1H, s, − N = CH), 13.97 (1H, s, SH). MS-FAB+: m/z: 319 (M++1). For C14H11FN4S2 calculated: 52.81 C, 3.48 H, 17.60 N; found: 52.96 C, 3.64 H, 17.67% N.
4-[(4-chlorobenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2c):
IR [ν, cm− 1, KBr]: 3339 (N–H), 2558 (SH), 1628 (C = N), 1535, 1265, 1053, 952 (N–C = S, amide I, II, III and IV bands), 718 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 4.32 (2H, s, CH2), 6.93–6.98 (2H, m, thiophene C3,4-H protons), 7.32–7.39 (1H, d J = 4.17 Hz, thiophene C5-H), 7.60–7.70 (2H, d J = 8.41 Hz, phenyl C2,6-H), 7.94–8.00 (2H, d J = 8.45 Hz, phenyl C3,5-H), 10.12 (1H, s, –N = CH), 13.89 (1H, brs, SH). MS-FAB+: m/z: 334 (M+), 335 (M++1), 336 (M++2). For C14H11ClN4S2 calculated: 50.22 C, 3.31 H, 16.73 N; found: 50.30 C, 3.22 H, 16.69% N.
4-[(4-methylbenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2d):
IR [ν, cm− 1, KBr]: 3354 (N–H), 2562 (SH), 1621 (C = N), 1533, 1261, 1050, 950 (N–C = S, amide I, II, III and IV bands), 709 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 2.35 (3H, s, CH3), 4.37 (2H, s, CH2), 6.92–7.40 (5H, m, aromatic protons), 7.73–7.80 (2H, d J = 8.06 Hz, phenyl C3,5-H), 9.92 (1H, s, –N = CH), 13.84 (1H, brs, SH). MS-FAB+: m/z: 314 (M+), 315 (M++1). For C15H14N4S2 calculated: 57.30 C, 4.49 H, 17.82 N; found: 57.34 C, 4.55 H, 17.84% N.
4-[(4-methoxybenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2e):
IR [ν, cm− 1, KBr]: 3357 (N–H), 2559 (SH), 1631 (C = N), 1538, 1260, 1049, 948 (N–C = S, amide I, II, III and IV bands), 712 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 3.85 (3H, s, OCH3), 4.36 (2H, s, CH2), 6.94–7.11 (2H, m, thiophene C3,4-H protons), 7.13–7.23 (2H, d J = 8.76 Hz, phenyl C2,6-H), 7.40–7.48 (1H, dd J = 5.04, 1.15 Hz, thiophene C5-H), 7.94–8.00 (2H, d J = 8.76 Hz, phenyl C3,5–H), 9.85 (1H, s, − N = CH), 13.85 (1H, brs, SH). MS-FAB+: m/z: 331 (M++1). For C15H14N4OS2 calculated: 54.52 C, 4.27 H, 16.96 N; found: 54.55 C, 4.25 H, 16.95% N.
4-[(4-cyanobenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2f):
IR [ν, cm− 1, KBr]: 3339 (N–H), 2566 (SH), 1605 (C = N), 1545, 1260, 1050, 950 (N–C = S, amide I, II, III and IV bands), 705 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 4.39 (2H, s, CH2), 6.95–7.10 (2H, m, thiophene C3,4-H protons), 7.40–7.50 (1H, dd J = 5.13, 1.13 Hz, thiophene C5-H), 8.00–8.10 (2H, d J = 8.37 Hz, phenyl C2,6-H), 8.12–8.20 (2H, d J = 8.35 Hz, phenyl C3,5-H), 10.50 (1H, s, − N = CH), 14.00 (1H, s, SH). MS-FAB+: m/z: 326 (M++1). For C15H11N5S2 calculated: 55.37 C, 3.41 H, 21.52 N; found: 55.49 C, 3.48 H, 21.48% N.
4-[(3,4-methylenedioxybenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2 g):
IR [ν, cm− 1, KBr]: 3329 (N–H), 2530 (SH), 1621 (C = N), 1535, 1263, 1052, 952 (N–C = S, amide I, II, III and IV bands), 713 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 4.40 (2H, s, CH2), 6.25 (2H, s, O–CH2–O), 6.98–7.59 (6H, m, aromatic protons), 9.90 (1H, s, –N = CH), 13.90 (1H, brs, SH). MS-FAB+: m/z: 345 (M++1). For C15H12N4O2S2 calculated: 52.31 C, 3.51 H, 16.27 N; found: 52.39 C, 3.55 H, 16.32% N.
4-[(3,4-dichlorobenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2 h):
IR [ν, cm− 1, KBr]: 3349 (N–H), 2558 (SH), 1625 (C = N), 1535, 1260, 1051, 950 (N–C = S, amide I, II, III and IV bands), 710 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 4.40 (2H, s, CH2), 6.95–8.20 (6H, m, aromatic protons), 10.20 (1H, s, –N = CH), 13.98 (1H, s, SH). MS-FAB+: m/z: 369 (M++1), 370 (M++2), 371 (M++3). For C14H10Cl2N4S2 calculated: 45.53 C, 2.73 H, 15.17 N; found: 45.65 C, 2.78 H, 15.21% N.
4-[(3-hydroxy-4-methoxybenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2i):
IR [ν, cm− 1, KBr]: 3359 (N–H), 3031 (OH), 2561 (SH), 1633 (C = N), 1533, 1261, 1050, 950 (N–C = S, amide I, II, III and IV bands), 719 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 3.86 (3H, s, OCH3), 4.30 (2H, s, CH2), 6.90–7.48 (6H, m, aromatic protons), 9.50 (1H, s, OH), 9.78 (1H, s, –N = CH), 13.77 (1H, s, SH). MS-FAB+: m/z: 346 (M+), 347 (M++1). For C15H14N4O2S2 calculated: 52.01 C, 4.07 H, 16.17 N; found: 51.94 C, 4.05 H, 16.14% N.
4-[(3,4-dihydroxybenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2j):
IR [ν, cm− 1, KBr]: 3354 (N–H), 3058 (OH), 2560 (SH), 1625 (C = N), 1535, 1262, 1052, 952 (N–C = S, amide I, II, III and IV bands), 717 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 4.37 (2H, s, CH2), 6.95–8.49 (6H, m, aromatic protons), 9.53 (2H, s, OH), 10.02 (1H, s, − N = CH), 13.96 (1H, s, SH). MS-FAB+: m/z: 333 (M++1). For C14H12N4O2S2 calculated: 50.59 C, 3.64 H, 16.85 N; found: 50.55 C, 3.60 H, 16.79% N.
4-[(3-nitro-4-hydroxybenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2k):
IR [ν, cm− 1, KBr]: 3361 (N–H), 3049 (OH), 2556 (SH), 1624 (C = N), 1535, 1264, 1053, 953 (N–C = S, amide I, II, III and IV bands), 715 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 4.38 (2H, s, CH2), 6.97–8.50 (6H, m, aromatic protons), 9.32 (1H, s, OH), 10.02 (1H, s, –N = CH), 13.96 (1H, s, SH). MS-FAB+: m/z: 362 (M++1). For C14H11N5O3S2 calculated: 46.53 C, 3.07 H, 19.38 N; found: 46.63 C, 3.05 H, 19.34% N.
4-[(4-methoxy-5-phenoxymethylbenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2 l):
IR [ν, cm− 1, KBr]: 3349 (N–H), 2566 (SH), 1615 (C = N), 1538, 1265, 1055, 955 (N–C = S, amide I, II, III and IV bands), 711 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 3.89 (3H, s, OCH3), 4.37 (2H, s, CH2), 5.21 (2H, s, OCH2), 6.92–7.68 (11H, m, aromatic protons), 9.88 (1H, s, –N = CH), 13.86 (1H, brs, SH). MS-FAB+: m/z: 437 (M++1). For C22H20N4O2S2 calculated: 60.53 C, 4.62 H, 12.83 N; found: 60.44 C, 4.65 H, 12.81% N.
4-[(3,4,5-trimethoxybenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2m):
IR [ν, cm− 1, KBr]: 3345 (N–H), 2548 (SH), 1620 (C = N), 1532, 1258, 1058, 957 (N–C = S, amide I, II, III and IV bands), 725 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 3.89 (9H, s, OCH3), 4.39 (2H, s, CH2), 6.92–7.03 (2H, m, thiophene C3,4-H protons), 7.32 (2H, s, phenyl C2,6-H), 7.41–7.50 (1H, dd J = 3.95, 1.14 Hz, thiophene C5-H), 10.04 (1H, s, –N = CH), 13.92 (1H, s, SH). MS-FAB+: m/z: 391 (M++1). For C17H18N4O3S2 calculated: 52.29 C, 4.65 H, 14.35 N; found: 52.31 C, 4.72 H, 14.38% N.
4-[(4-dimethylamino-6-chlorobenzylidene)amino]-5-(2-thienylmethyl)-4H-1,2,4-triazole-3-thiol (2n):
IR [ν, cm− 1, KBr]: 3347 (N–H), 2559 (SH), 1618 (C = N), 1531, 1266, 1052, 954 (N–C = S, amide I, II, III and IV bands), 719 (C–S–C of thiophene). 1H-NMR (250 MHz, δ ppm, DMSO-d6,): 3.13 (6H, s, N(CH3)2), 4.39 (2H, s, CH2), 6.78–8.10 (6H, m, aromatic protons), 10.34 (1H, s, –N = CH), 13.83 (1H, s, SH). MS-FAB+: m/z: 378 (M++1), 380 (M++3). For C16H16ClN5S2 calculated: 50.85 C, 4.27 H, 18.53 N; found: 50.91 C, 4.30 H, 18.64% N.
Microbiology
In-vitro evaluation of antimycobacterial activity against mycobacterium tuberculosis H37Rv
Antitubercular activities of the compounds were tested at the center of Tuberculosis Antimicrobial Acquisiton & Coordinating Facility (TAACF). Compounds were tested for in-vitro antituberculosis activity against Mycobacterium tuberculosis H37Rv (ATCC 27294) at 6.25 μg/mL, in BACTEC 12B medium using a broth microdilution assay and the Microplate Alamar Blue Assay (MABA). Compounds exhibiting fluorescence were tested in the BACTEC 460 Radiometric System [Citation9].
BACTEC radiometric method of susceptibility testing
Inocula for susceptibility testing were either from a positive BACTEC isolation vial with a growth index (GI) of 500 more, or suspension of organism isolated earlier on conventional medium. The culture was well mixed with a syringe and 0.1 mL of a positive BACTEC culture was added to each of the vials containing the test drugs. A drug vial contained rifampicin (0.25 μg/mL) was used as reference antituberculosis agent. A control vial was inoculated with a 1:100 microdilution of the culture. A suspension equivalent to a McFarland No.1 standard was prepared in the same manner as a BACTEC positive vial, when growth from a solid medium was used. Each vial was tested immediately on a BACTEC instrument to provide CO2 in the headspace. The vials were incubated at 37°C and tested daily with a BACTEC instrument. When the GI in the control read at least 30, the increase in GI (ΔGI) from the previous day in the control was compared with that in the drug vial. The following formulae were used to interpret results:
If a clear susceptibility pattern (the difference of ΔGI of control and the drug bottle) was not seen at the time the control ΔGI is 30, the vials were read for 1 or 2 additional days to establish a definite pattern of ΔGI differences.
Results and discussion
In the present work, 14 new compounds were synthesized. The synthesic route for the compounds is outlined in Scheme . For the synthesis of the title compounds, 4-amino-3-mercapto-5-(2-thienylmethyl)-1,2,4-triazole (1) required as starting material was prepared for the first time by the reaction of thienyl-2-acetic acid with thiocarbohydrazide Citation10-12. The reaction of equimolar quantities of these triazoles (1) with respective arylaldehydes in the presence of concentrated sulfuric acid in dioxane [Citation12] resulted in the formation of the title compounds (2a–n) ().
The structures of the obtained compounds were elucidated by spectral data. In the IR spectra, some significant stretching bands due N–H, S–H, C = N and N–C = S were at 3361–3328 cm− 1, 2568–2525 cm− 1, 1633–1605 cm− 1 and 1545–950 cm− 1 respectively. The specific band of thiophene was observed at 725–705 cm− 1. Compounds 2a–n exist as thiol-thione tautomers as indicated by their IR spectra which showed a band due to SH and four bands due to N–C = S I, II, III, IV. In the 1H-NMR spectra, the signal due to Ar-CH2-Ar methylene protons, present in all compounds, appeared at 4.30–4.40 ppm as a singlet. The azomethine derivatives 2a–n were characterized by the presence of the methine protons–N = CH– at 9.78–10.50 ppm as a singlet. The S–H proton was observed at 13.77–14.00 ppm as a singlet or broad singlet. All the other aromatic and aliphatic protons were observed in the expected regions. Mass spectra (MS (FAB)) of compounds showed M++1 peaks in agreement with their molecular formula. All compounds gave satisfactory elemental analysis.
The antituberculosis activities of the synthesized compounds were screened in-vitro using a BACTEC 460 radiometric system against Mycobacterium tuberculosis H37Rv (ATCC 27294) at 6.25 μg/mL. The results of the biological evaluation, expressed as a percentage inhibition of the growth of mycobacterium, are summarized in , and for the sake of comparison, the % inhibition for rifampicin, used as reference, is also included (Dose used = 0.25 μg/mL). All tested compounds proved to be less active than rifampicin against Mycobacterium tuberculosis H37Rv. The activity was affected by substituents on the phenyl ring. Thus, compound 2k which including nitro-groups and hydroxyl groups on the phenyl showed the highest inhibition (87%). Other compounds showed varying degrees of inhibition between 36–69%. We concluded from our investigations that 2k may be considered promising for the development of new antituberculosis agents.
Table II. Antituberculosis activity of the compounds.
Acknowledgements
The Authors are thankful to the Tuberculosis Antimicrobial Acquisition & Coordinating Facility (TAACF) in the USA for the in vitro evaluation of antimycobacterial activity.
References
- Ryan F. The forgotten plague. How the battle against tuberculosis was won and lost, p. 3. Little, Brown, Boston 1993; 460
- World Health Organization. The World Health Organization Global Tuberculosis Program. http://www.who.int/gtb/. 2003.
- Kimerling ME, Kluge H, Vezhnina N, Iacovazzi T, Demeulenaere T, et al. Inadequacy of the current WHO re-treatment regiment in central Siberian prisons: treatment failure and MDR-TB. Int J Tuberc Lung Dis 1999; 3: 451–453
- De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis 1999; 3: 457–465
- Tuberculosis: a global emergency. World Health Forum 1993; 14: 438.
- Global Alliance for Tuberculosis Drug Development. Scientific blueprint for TB drug development. Tuberculosis 2001;81(Suppl.): 1–52.
- Zhang Y. The magic bullets and tuberculosis drug targets. Annu Rev Pharmacol Toxicol 2005; 45: 529–564
- Kaplancıklı ZA, Turan-Zitouni G, Chevallet P. Synthesis and antituberculosis activity of new 3-alkylsulfanyl-1,2,4-triazole derivatives. J Eny Inhib Med Chem 2005; 20: 179–182
- Collins L, Franzblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. 1997; 41: 1004–1009, Antimicrob Agents Chemother
- Masao K, Hiroshi T. 1,2,4-Triazole derivatives. Jpn. Pat, 21, (1986) 420, Chem. Abst. 1986; 105: 642097.
- Turan-Zitouni G, Kaplancıklı ZA, Kılıç FS, Erol K. The synthesis of some triazolylphenothiazine derivatives and their antidepressant and anxiolytic activities. Boll Chim Farm 2002; 141: 192–196
- Turan-Zitouni G, Kaplancıklı ZA, Erol K, Kılıç FS. Synthesis and analgesic activity of some triazoles and triazolothiadiazines. Il Farmaco 1999; 54: 218–223