Abstract
A convenient method for the ‘one-pot’ synthesis of novel target molecule 2,7-diaryl-[1,4]-diazepan-5-ones from the respective 2,6-diaryl-piperidin-4-ones was catalyzed by NaHSO4.Al2O3 heterogeneous catalyst in dry media under microwave irradiation in solvent-free conditions. Moreover, the catalyst could be recovered and re-used up to 4 times after washing with ethyl acetate. They were evaluated for potential antibacterial activity against Staphylococcus aureus, β-Haemolytic streptococcus, Vibreo cholerae, Salmonella typhii, Escherichia coli, Klebsiella pneumonia, Pseudomonas and antifungal activity against Aspergillus flavus, Aspergillus fumigatus, Mucor, Candida albicans and Rhizopus. Structure-Activity Relationship (SAR) led to the conclusion that, of all the compounds 25–32 tested, compound 30 exerted strong in vitro antibacterial activity against S. aureus, S. typhii, and Pseudomonas and all the compounds 25–32 were less active against E. coli, whereas all the compounds 25–32 displayed potent in vitro antifungal activity against all the fungal strains used, except compound 30, which was more effectual against Mucor.
Introduction
Over the past decades, the incidence of systemic microbial infections has been increasing dramatically due to an increase in the number of immuno-compromised hosts [Citation1]. The increasing incidence of bacterial resistance to a large number of antibacterial agents such as glycopeptides (vancomycin, inhibition cell walls synthesis), sulfonamide drugs (inhibitors of tetrahydrofolate synthesis), β-lactam antibiotics (penicillins and cephalosporins), nitroimidazoles and quinolones (DNA inhibitors), tetracyclins, chloramphenicol and macrolides (erythromycin, inhibiting protein synthesis) is becoming a major concern [Citation2]. For the past several years, vancomycin has been considered the last line of defense as an agent against Gram-positive infections and no alternative drugs are available for treating diseases that have become resistant to vancomycin [Citation3]. Patients undergoing organ transplants, anticancer chemotherapy or long term treatment with antimicrobial agents and patients with AIDS are immunosuppressed and very susceptible to life threatening systemic fungal infections such as Candidiasis, cryptococcosis and aspergillosis. Antifungal azoles, fluconazole and itraconazole which are strong orally active inhibitors of lanosterol 14a-demethylase (cytochrome P45014DM) have been widely used in antifungal chemotherapy. Reports are available on the developments of resistance to currently available antifungal azoles in Candida spp., as well as clinical failures in the treatment of fungal infections Citation4-7. Furthermore, most of the present antifungal drugs are not effective against invasive aspergillosis and the only drug of choice in such patients is the injectable amphotericin B. Some examples of 1,2,4-triazole based antibacterial and antifungal drugs are estazolam [Citation8,Citation9], alprazolam [Citation10] and rizatriptane [Citation11]. These observations place new emphasis on the need to search for alternative new and more effective antimicrobial agents with a broad spectrum. Recently, we expanded the synthesis of 2,6-diarylpiperidin-4-one derivatives Citation12-16 with a view to incorporating various other bioactive heterocyclic nucleui such as selenadiazoles, and thiadiazoles for evaluation of their associated antibacterial and antifungal activities. Synthesis of molecules, which are novel yet still resembling known biologically active molecules by virtue of the presence of some critical structural features, is an essential component in the search for new leads in a drug design programme. Antibiotics such as penicillins and cephalosporins have an amide group. Novel bioactive natural compounds [Citation17] are synthesized by the conversion of ketones into amides, since the amide group is an important pharmacophore. Consequently in the interest of above, we planned to synthesize the novel bioactive target molecule, 2,7-diaryl-[1,4]-diazepan-5-one, which has the key functional amide group present.
Microwave irradiation (MWI) has become an established tool in organic synthesis, because of the rate enhancements, higher yields, and often, improved selectivity, with respect to conventional reaction conditions Citation18-23. In recent years, solvent –free reactions using either organic or inorganic solid supports have received increasing attention [Citation24]. There are several advantages to performing synthesis in dry media: (i) short reaction times, (ii) increased safety, (iii) economic advantages due to the absence of solvent. In addition, solvent free MWI processes are also clean and efficient.
The challenge in chemistry to develop practical processes, reaction media, conditions and/or utility of materials based on the concept of green chemistry is one of the important issues in the scientific community [Citation25]. Owing to our interest in synthesizing fascinating pharmacological and therapeutically important compounds under solid-state reaction conditions Citation26-28, we attempted and succeeded in using neutral alumina supported sodium hydrogen sulphate (NaHSO4.Al2O3) [Citation29], as a heterogeneous catalyst for the one-pot conversion of ketones to amides. This prompted us to continue our work on the application of NaHSO4.Al2O3 heterogeneous catalyst for the ‘one-pot’ synthesis of the novel target molecule 2,7-diaryl-[1,4]-diazepan-5-ones from the respective 2,6-diaryl-piperidin-4-ones in dry media under microwave irradiation in solvent-free conditions.
Experimental
Microbiology
Materials
All the bacterial strains namely Staphylococcus aureus, β-Haemolytic streptococcus, Vibreo cholerae, Salmonella typhii, Escherichia coli, Klebsiella pneumonia, Pseudomonas and fungal strains namely Aspergillus flavus, Aspergillus fumigatus, Mucor, Candida albicans, Rhizopus were obtained from the Faculty of Medicine, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India.
In vitro antibacterial and antifungal activity
The in vitro activities of the compounds were tested in Sabourauds dextrose broth (SDB) (Hi-media, Mumbai) for fungi and nutrient broth (NB) (Hi-media, Mumbai) for bacteria by the Disc Diffusion method [Citation33]. The respective hydrochlorides of the test compounds 25–32 were dissolved in water to obtain 1 mg mL− 1 stock solution and different concentrations (100, 200, 500 ppm) were prepared. Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1 to 7 days old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in SDB. Sterile paper disc of 5 mm diameter were saturated with the three different concentrations and placed in each seeded agar plate. The petri plates were incubated in a BOD incubator at 37°C for bacteria and at 28°C for fungi. The zone of inhibition was recorded by visual observations after 24 h inhibition for bacteria and after 72–96 h inhibition for fungi. Moreover, the zone of inhibition was measured by excluding the diameter of the paper disc. Ciprofloxacin was used as a standard for bacteria and fluconazole for fungi under analogous conditions.
Chemistry
TLC was used to assess the reactions and the purity of the products. Melting points were taken in open capillaries and were uncorrected. IR spectra were recorded in KBr (pellet) on a Nicolet-Avatar–360 FT-IR spectrophotometer and noteworthy absorption values (cm− 1) alone are listed. 1H-NMR spectra were recorded at 300 MHz on a Bruker AV 300 spectrometer using CDCl3 as solvent and TMS as internal standard. The APCI + ve mass spectra were recorded on a Shimazdu LCMS-QP8000α LC MS spectrometer. Satisfactory microanalysis was obtained on a Carlo Erba 1106 CHN analyzer. A conventional (unmodified) domestic microwave oven equipped with a turntable (LG, MG-395 WA, 230V ∼ 50 Hz, 760 W) was used for the irradiation. By adopting the literature precedent [Citation34], 2,6-diarylpiperidin-4-ones (9–16) were prepared by the condensation of the appropriate ketones, aldehydes and ammonium acetate in a 1:2:1 ratio.
Preparation of 2,7-diphenyl-[1,4]-diazepan-5-one (25)
In a 100 mL borosil beaker, 2,6-diphenylpiperidin-4-one (9) (0.1 mol) and NH2OH.HCl (0.3 mmol) were mixed thoroughly with NaHSO4.Al2O3 catalyst (150 mg), and the reactions were irradiated at 320W for 150 s. The mixture was removed from the oven, cooled and shaken with ethyl acetate (40 mL) and the catalyst removed by filtration. The filtrate was concentrated and the residue was subjected to column chromatography over silica gel using benzene [CARE-CARCINOGEN]:CHCl3 (1.2:0.8) as eluent to afford the corresponding product (25) as a crystalline solid. IR (KBr) (cm− 1): 3447, 3311, 3082, 2927, 2792, 1671; MS (m/z): 267 (M+) (M.F. C17H18N2O); 1H NMR (δ ppm): 2.11 (s, 1H, H1), 2.65–2.69 (d, 1H, H6e, J = − 14.10), 3.00–3.34 (m, 2H, H3e & H6a), 3.58–3.82 (m, 1H, H3a), 4.02–4.05 (d, 2H, H2, J = 9.0), 4.12–4.16 (d, 2H, H7, J = 10.2), 6.16 (s, 1H, H4), 7.26–7.45 (m, 10H, Harom.).
Compounds 26–32 were synthesized similarly.
3-Methyl-2,7-diphenyl-[1,4]-diazepan-5-one (26)
IR (KBr) (cm− 1): 3302, 3207, 3082, 2925, 2880, 2852, 1667; MS (m/z): 281 M+ (M.F. C18H20N2O); 1H NMR (δ ppm): 0.61–0.90 (d, 3H, CH3), 2.07 (s, 1H, H1), 2.63–2.68 (d, 1H, H6e, J = − 14.1), 3.11–3.19 (t, 1H, H6a), 3.81–3.84 (m, 1H, H3a), 3.69–3.71 (d, 2H, H2, J = 7.8), 4.12–4.15 (d, 2H, H7, J = 10.8), 5.82 (s, 1H, H4), 7.26–7.38 (m, 10H, Harom.).
3-Ethyl-2,7-diphenyl-[1,4]-diazepan-5-one (27)
IR (KBr) (cm− 1): 3308, 3224, 3061, 2978, 2929, 2881, 2852, 1663; MS (m/z): 295 M+ (M.F. C19H22N2O); 1H NMR (δ ppm): 0.61–0.89 (t, 3H, CH3), 1.01–1.21 (m, 2H, CH2), 2.03 (s, 1H, H1), 2.63–2.67 (d, 1H, H6e, J = − 13.8), 3.11–3.18 (t, 1H, H6a), 3.61–3.69 (m, 1H, H3a), 3.75–3.79 (d, 2H, H2, J = 7.5), 4.12–4.15 (d, 2H, H7, J = 10.5), 5.76 (s, 1H, H4), 7.21–7.42 (m, 10H, Harom.).
3-Isopropyl-2,7-diphenyl-[1,4]-diazepan-5-one (28)
IR (KBr) (cm− 1): 3590, 3399, 3060, 2972, 2847, 2840, 2837, 1650; MS (m/z): 309 M+ (M.F. C20H24N2O); 1H NMR (δ ppm): 0.93–0.97 (d, 6H, CH3), 1.58–1.62 (m, 1H, CH), 2.07 (s, 1H, H1), 2.62–2.66 (d, 1H, H6e, J = − 14.2), 3.14–3.18 (t, 1H, H6a), 3.59–3.63 (m, 1H, H3a), 3.93–3.97 (d, 2H, H2, J = 7.6), 4.08–4.12 (d, 2H, H7, J = 10.4), 5.74 (s, 1H, H4), 7.28–7.45 (m, 10H, Harom.).
3,6-Dimethyl-2,7-diphenyl-[1,4]-diazepan-5-one (29)
IR (KBr) (cm− 1): 3446, 3333, 3081, 2979, 2934, 2821, 1661; MS (m/z): 295 M+ (M.F. C19H22N2O); 1H NMR (δ ppm): 0.72–0.82 (d, 6H, CH3), 2.08 (s, 1H, H1), 3.05–3.15 (m, 1H, H6e), 3.87–3.91 (m, 1H, H3a), 3.64–3.68 (d, 2H, H2, J = 7.9), 3.79–3.81 (d, 2H, H7, J = 8.9), 5.69 (s, 1H, H4), 7.21–7.38 (m, 10H, Harom.).
2,7-Bis(2-chloro-phenyl)-[1,4]-diazepan-5-one (30)
IR (KBr) (cm− 1): 3449, 3316, 3089, 2932, 2798, 1675; MS (m/z): 336 (M+) (M.F. C17H16Cl2N2O); 1H NMR (δ ppm): 2.18 (s, 1H, H1), 2.68–2.72 (d, 1H, H6e, J = − 14.2), 3.26–3.30 (m, 2H, H3e & H6a), 3.79–3.83 (m, 1H, H3a), 4.57–4.61 (d, 2H, H2, J = 8.9), 4.68–4.72 (d, 2H, H7, J = 10.2), 6.21 (s, 1H, H4), 7.40–7.81 (m, 8H, Harom.).
2,7-Bis(2-chloro-phenyl)-3-methyl[1,4]-diazepan-5-one (31)
IR (KBr) (cm− 1): 3308, 3212, 3087, 2928, 2885, 2857, 1669; MS (m/z): 350 M+ (M.F. C18H18Cl2N2O); 1H NMR (δ ppm): 0.91–0.99 (d, 3H, CH3), 2.17 (s, 1H, H1), 2.69–2.73 (d, 1H, H6e, J = − 14.2), 3.20–3.24 (t, 1H, H6a), 3.90–3.94 (m, 1H, H3a), 4.49–4.53 (d, 2H, H2, J = 7.6), 4.70–4.74 (d, 2H, H7, J = 10.5), 5.89 (s, 1H, H4), 7.41–7.70 (m, 8H, Harom.).
2,7-Bis(2-chloro-phenyl)-3,6-dimethyl[1,4]-diazepan-5-one (32)
IR (KBr) (cm− 1): 3449, 3337, 3089, 2983, 2939, 2827, 1669; MS (m/z):364 M+ (M.F. C19H20Cl2N2O); 1H NMR (δ ppm): 0.85–0.95 (d, 6H, CH3), 2.18 (s, 1H, H1), 3.19–3.23 (m, 1H, H6e), 3.93–3.97 (m, 1H, H3a), 4.49–4.53 (d, 2H, H2, J = 7.8), 4.61–4.65 (d, 2H, H7, J = 8.7), 5.79 (s, 1H, H4), 7.41–7.80 (m, 8H, Harom.).
Chemistry
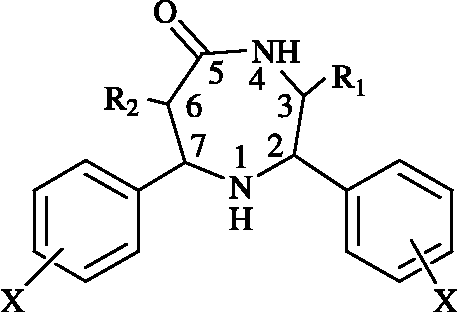
Treatment of 2,6-diarylpiperidin-4-ones with hydroxylamine hydrochloride together with a catalytic amount of NaHSO4.Al2O3 afforded the corresponding 2,7-diaryl-[1,4]-diazepan-5-ones in high yields in dry media under MW irradiation without any of the environmental disadvantages of using toxic reagents like sodium azide [Citation30]. The NaHSO4.Al2O3 catalyst was shown to be one of the most efficient MW absorbers with a very high specificity to MW heating and was able to reach a temperature of 120°C after 5 minutes irradiation in a domestic oven (320 W). The 1:1 ratio of NaHSO4.Al2O3 catalyst to substrate is the most acceptable ratio in terms of efficiency and safety; a power level of 320 watts is the most suitable one. The product was separated using ethyl acetate and purified by column chromatography. The schematic representation and analytical data for the synthesized compounds 25–32 are given in Scheme and , respectively.
Table I. Reaction conditions and analytical data for compounds 25–32a.
The conversion of 2,6-diarylpiperidin-4-ones into 2,7-diaryl-[1,4]-diazepan-5-ones by this method is believed to follow the Beckmann rearrangement. In the first step, 2,6-diarylpiperidin-4-ones are converted to their respective oximes and rapidly rearrange to give 2,7-diaryl-[1,4]-diazepan-5-ones in the second step. The attempt to isolate the respective oximes from the reaction mixture was unsuccessful.
The formations of 2,7-diaryl-[1,4]-diazepan-5-ones via the oximes was confirmed by the same kind of reactions carried out using NaHSO4.Al2O3 catalyst and 2,6-diarylpiperidin-4-one oximes (17–24) [Citation31], under microwave irradiation. The products formed from the above two methods were found to be identical.
Re-use of NaHSO4.Al2O3 heterogeneous catalyst in the synthesis of 3-methyl-2,7-diphenyl-[1,4]-diazepan-5-one (26)
NaHSO4.Al2O3 heterogeneous catalyst can be recovered and re-used up to four times () by washing with ethyl acetate after each use and then activating it in an oven at 120°C for 1 h prior to use, thus rendering the process more economical and green. ().
Results and discussion
Antibacterial activity
All the synthesized novel target molecule 2,7-diaryl-[1,4]-diazepan-5-ones 25–32 were tested for their antibacterial activity in vitro against Staphylococcus aureus, β-H. streptococcus, V. cholerae, S. typhii, E. coli, K. pneumonia and Pseudomonas. Ciprofloxacin was used as a standard drug. The zone of inhibition (mm) values are shown in .
Table II. In vitro antibacterial activity of compounds 25–32.
In general all the synthesized novel 2,7-diaryl-[1,4]-diazepan-5-ones 25–32 exerted a wide range of modest antibacterial activity in vitro against the tested organisms, their activity decreasing upon dilution. All the compounds were less active against Escherichia coli and more active against Staphylococcus aureus, Salmonella typhii and Pseudomonas. Introduction of an alkyl groups at C3/C6 position in compounds 26–29, 31 and 32 did not influence much the biological activities. However, among the chloro-substituted compounds 30–32, 2,7-bis(2-chloro-phenyl)-[1,4]-diazepan-5-one (30), was the most effective compound.
Antifungal activity
The in vitro antifungal activity of the synthesized novel heterocyclic compounds 25–32 was studied against certain fungal strains viz., A. flavus, A. fumigatus, Mucor, C. albicans and Rhizopus. Fluconazole was used as a standard drug. The zone of inhibition (mm) values are shown in . Generally all the synthesized compounds exerted a wide range of modest in vitro antifungal activity against all the tested organisms, their activity decreasing upon dilution. Of the chloro-substituted compounds 30–32, 2,7-bis(2-chloro-phenyl)-[1,4]-diazepan-5-one (30) was the most active compound. Moreover, of all the compounds tested, 30 was the most effective against Mucor.
Table III. In vitro antifungal activity of compounds 25–32.
Conclusion
Examination of the in vitro antibacterial and antifungal activity profiles for the differently substituted novel 2,7-diaryl-[1,4]-diazepan-5-ones against the tested bacterial and fungal strains, provides a structure-activity relationship, which may be summarized as follows:
The nature of the substituent on the phenyl ring viz., chloro moiety is determinant for the nature and extent of the activity of the synthesized compounds, which might an influence on their inhibiting mechanism of action. Although this is unknown, these observations may promote further developments of our research in this field. Which may lead to compounds with a better pharmacological profile than standard drugs and serve as templates for the construction of better drugs to combat bacterial and fungal infection.
Acknowledgements
The Authors are thankful to Professor K. Pandiarajan, Head, Department of Chemistry, Annamalai University for providing the necessary facilities. P. Sureshkumar, V. Kanagarajan and J. Thanusu are highly thankful to the Annamalai University authorities for providing financial support in the form of Research Fellowship.
References
- Davies J. Bacteria on the rampage. Nature 1996; 383: 219
- Chu DTW, Plattner JJ, Katz L. New directions in antibacterial research. J Med Chem 1996; 39: 3853
- Spera RV, Jr, Farber BF. Multidrug resistant enterococcus faecium. an untreatable nosocomial pathogen. Drugs 1994; 48: 678
- Odds FC. Resistance of yeasts to azole-derivative antifungals. J Antimicrob Chemother 1993; 31: 463
- Hitchock CA. Mechanism of fluconazole resistance in candida albicans isolates from Japanese AIDS patients. Biochem Soc Trans 1993; 21: 10
- Johnson EM, Warnock DW, Luker J, Porter SR. Emergence of azole drug resistance in candida species from HIV infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother 1995; 35: 103
- Rex JH, Rinaldi MG, Pfaller MA. Resistance of candida species to fluconazole. Antimicrob Agents Chemother 1995; 39: 1
- The Merck Index & Co, Inc. Whitehouse station, NJ, USA 2001; 3737
- Zajac M, Pawelczyk E, Chemia Lekow AM. Poznan 2000; 188
- The Merck Index & Co, Inc. Whitehouse station, NJ, USA 2001. p 320.
- The Merck Index & Co, Inc. Whitehouse station, NJ, USA 2001. p 8324.
- Balasankar T, Gopalakrishnan M, Nagarajan S. Synthesis and antibacterial activity of some 5-(4-biphenylyl)-7-aryl[3,4-d][1,2,3]-benzothiadiazoles. Eur J Med Chem 2005; 40: 728
- Suganya V. 1999, Annamalai University, India, Synthesis and spectral studies of some 2,6-diaryl-N-hydroxypiperidin-4-ones. Ph D Thesis
- Baskar G. 2001, Ph D Thesis, Annamalai University, India, Synthesis and spectral analysis of some N-hydroxypiperidones and 1,3-thiazines
- Winfred Jebaraj J. 2001, Synthesis and spectral studies of some 1,2,3-selenadiazoles and 1,2,3-thiadiazoles. Ph D Thesis, Annamalai University, India
- Manikandan H. 2002, Synthesis and antimicrobial activity of some nitrogen and sulfur containing heterocycles. Ph D Thesis. Annamalai University, India
- Marouka K, Yamamoto H. Comprehensive Organic Synthesis. Pergamonn Press, Trost B M 1991; 6: 6221
- Caddick S. Microwave assisted organic reactions. Tetrahedron 1995; 95: 10403
- Deshayes S, Liagre M, Loupy A, Luche J, Petit A. Microwave activation in phase transfer catalysis Tetrahedron 1999; 55: 10851
- Lidstrom P, Tierney J, Wathey B, Westman J. Microwave assisted organic synthesis- A review. Tetrahedron 2001; 57: 9225
- Kirschning A, Monenschein H, Wittenberg R. Functionalized polymers-Emerging versatile tools for the solution-phase chemistry and automated parallel synthesis. Angew Chem Int Ed 2001; 113: 670
- Varma RS. Solvent-free accelerated organic syntheses using microwaves. Pure Appl Chem 2001; 73: 193
- Loupy A. Microwaves in Organic Synthesis. Wiley-VCH, Weinheim 2002
- Diddams P, Butters M. In Solid Supports and Catalysts in Organic Synthesis. Smith K, Ellis Harwood and PTR Prentice Hall, New York and London 1992, Chapters 1, 3 and 5
- Tundo P, Anastas PT. Green Chemistry: Challenging Perspectives. Oxford Science, Oxford 1999
- Gopalakrishnan M, Anandabaskar T, Sureshkumar P, Thanusu J, Kumaran AK, Kanagarajan V. Phase-transfer catalysis for the synthesis of hydroxylamines from oximes using benzyltriethylammonium borohydride in methanol and under solid-phase conditions. Mendeleev Commun 2006; 1: 51
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J, Govindaraju R. Silica gel supported sodium hydrogen sulfate as an efficient and reusable heterogeneous catalyst for the synthesis of imines in solvent-free conditions under microwave irradiation. J Chem Res 2005; 5: 299
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Aluminium metal powder (atomized) catalyzed Friedel-crafts acylation in solvent-free conditions: A facile and rapid synthesis of aryl ketones under microwave irradiation. Catal Commun 2005; 6: 753
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Organic synthesis in solid media. Alumina supported sodium hydrogen sulfate as an effective and reusable catalyst for ‘one-pot’ synthesis of amides from ketones in dry media under microwave irradiation. Lett Org Chem 2005; 2: 136
- Sabapathymohan RT. Synthesis of some novel heterocyclic compounds. Ph D Thesis. Annamalai University, India 1986
- Gopalakrishnan M, Pazhamalai S, Sureshkumar P, Thanusu J, Kanagarajan V. Microwave assisted Oximation reaction using CaO as a catalyst under solvent-less conditions. E.J. Chem 2007 (Communicated).
- Baliah V, Lakshmanan Mr, Pandiarajan K. Synthesis of some 1,2-ethanediamines. Indian J Chem 1978; 16B: 72
- Maruzella JC, Percival AH. Antibacterial activity of essential oil vapors. J Am Pharm Assoc 1958; 47: 471
- Noller CR, Baliah V. The preparation of some piperidine derivatives by the Mannich reaction. J Am Chem Soc 1948; 70: 3853
![Scheme 1 A convenient synthesis of some novel 2,7-diaryl-[1,4]-diazepan-5-ones (25–32).](/cms/asset/553b5b4a-2247-4fe7-a63c-ec51ebf020cf/ienz_a_226970_f0001_b.gif)
![Figure 1 Re-use of NaHSO4.Al2O3 in the synthesis of 3-methyl-2,7-diphenyl-[1,4]-diazepan-5-one (26). The reaction was carried out in a domestic microwave oven operating at 320W.](/cms/asset/8356a873-6430-4d67-85de-63117cf19f12/ienz_a_226970_f0002_b.gif)