Abstract
Inhibitory effects of some antibiotics on purified human erythrocyte glutathione reductase were investigated. Human erythrocyte glutathione reductase was purified 2800-fold (29% yield) at 4°C using 2′, 5′-ADP Sepharose 4B affinity gel and Sephadex G-200 gel filtration chromatography. SDS polyacrylamide gel electrophoresis showed a single band for the enzyme. Imipenem, rifamycin, sulfanylacetamide, ceftazidime, chloramphenicol, seftriaxon, vancomycin, cefuroxime and ornidazole exhibited inhibitory effects but clindamycin, lincomycin, amoxicillin, amikacin exhibited activatory effects on the enzyme in vitro. The IC50 values of imipenem, rifamycin, sulfanylacetamide, ceftazidime, chloramphenicol, seftriaxon, vancomycin, cefuroxime and ornidazole were 0.030, 0.146, 0.59, 2.476, 2.36, 2.88, 4.83, 15.43 and 19.632 mM, respectively, and the Ki constants were 0.06 ± 0.01, 0.275 ± 0.10, 0.85 ± 0.05, 3.59 ± 0.51, 3.85 ± 0.40, 3.71 ± 0.60, 15.11 ± 2.50, 23.50 ± 2.94 and 28.49 ± 6.50 mM, respectively. While imipenem, rifamycin, sulfanylacetamide, ceftazidime, chloramphenicol and seftriaxon cefuroxime and ornidazole showed competitive inhibition, vankomycine displayed noncompetitive inhibition.
Introduction
Glutathione (γ-L-glutamyl-L-cysteinylglycine; GSH) has many important functions: It is an antioxidant, is involved in the detoxification of xenobiotics, and serves as a cofactor in isomerization reactions [Citation1]. Glutathione has an important role in the synthesis and degradation of proteins, regulation of enzymes, formation of the deoxyribonucleotid precursors of deoxyribonucleic acid (DNA) and protection of cells against free radicals and reactive oxygen species [Citation2]. Responsible for the reduction of GSSG to GSH is the enzyme glutathione reductase (GR; NADPH: oxidized glutathione oxidoreductase, EC 1.6.4.2), a flavoprotein which uses NADPH as electron donor for the reduction reaction. GR enables several vital functions of the cell, such as the detoxification of free radicals and reactive oxygen species as well as protein and DNA biosynthesis, by maintaining a high ratio of GSH/GSSG [Citation3]. Decreased glutathione levels have also been reported in several diseases, such as acquired immune deficiency syndrome (AIDS) [Citation4], Parkinson's disease [Citation5] and diabetes [Citation6,Citation7]. A major role of GSH in erythrocytes is the prevention of hemoglobin denaturation, preserving the integrity of erythrocyte membrane sulfhydryl groups and detoxification of the xenobiotics and reactive oxygen species in red blood cells [Citation8]. GR has been purified from erythrocytes, using different purification procedures all of which involve several chromotographic steps Citation9-14. The effects of many commonly used drugs on human, sheep and rat erythrocytes GR enzyme activities have been investigated Citation15-18. However, no reports could be found in the literature on the effects of imipenem, rifamycin, sulfanylacetamide, ceftazidime, chloramphenicol, seftriaxon, vancomycin, cefuroxime and ornidazole on human erythrocyte GR.
The aim of this study was purifying human erythrocyte GR and the determination of inhibition or activation effects of some antibiotics on the purified GR.
Materials and methods
Materials
Sephadex G-200, NADPH, GSSG, protein assay reagents and chemicals for electrophoresis were obtained from Sigma Chem. Co. 2′, 5′-ADP Sepharose-4B was obtained from Pharmacia. All other chemicals used were of analytical grade and obtained from either Sigma-Aldrich or Merck.
Activity determination
Enzymatic activity was measured by Beutler's method [Citation19]. One enzyme unit is defined as the oxidation of 1 μmol NADPH per min under the assay condition at 25°C, pH 8.0.
Preparation of the hemolysate
Fresh human blood samples were collected in tubes containing EDTA, then centrifuged (15 min, 2,500 × g) and plasma and buffy coat (leucocytes) were removed. The packed red cells were washed three times with KCl (0.16 M), hemolyzed with 5 volume of ice-cold water and then centrifuged (48oC, 10,000 × g, for 30 min) to remove the ghosts and intact cells [Citation15].
Ammonium sulphate precipitation
The hemolysate was subjected to precipitation with ammonium sulphate (between 30% and 70%). Enzyme activity was determined both in the supernatant and in the precipitate for each respective precipitation. The precipitate was dissolved in phosphate buffer (50 mM, pH = 7.0). The resultant solution was clear, and contained partially purified enzyme. This solution was dialysed at 4°C in 1 mM EDTA +10 mM K-phosphate buffer (pH 7.5) for 2 h with two changes of buffer [Citation15]. Partially purified enzyme solution was kept at 4°C.
Purification of the glutathione reductase
2′, 5′-ADP Sepharose-4B affinity chromatography
Two grams of dried 2′, 5′-ADP Sepharose-4B was used for a column (1 × 10 cm) of 10 mL bed volume. The gel was washed with 300 mL of distilled water to remove foreign bodies and air, suspended in 0.1 M K-acetate +0.1 M K-phosphate buffer (pH 6.0), and packed in the column. After settling of the gel, the column was equilibrated with 50 mM K-phosphate buffer including 1 mM EDTA, pH 6.0, by means of a peristaltic pump. The flow rates for washing and equilibration were adjusted to 20 mL/h. The dialyzed sample obtained previously was loaded onto the 2′, 5′-ADP Sepharose-4B affinity column and the column was washed with 25 ml of 0.1 M K-acetate +0.1 M K-phosphate, pH 6, and 25 ml of 0.1 M K-acetate +0.1 M K-phosphate, pH 7.85. Washing was continued with 50 mM K-phosphate buffer including 1 mM EDTA, pH 7.0, until the final absorbance difference became 0.05 at 280 nm. The enzyme was eluted with a gradient mixture of 0 to 0.5 mM GSH +1 mM NADPH in 50 mM K-phosphate, containing 1 mM EDTA (pH 7.0). Active fractions were collected and dialyzed with equilibration buffer. All of the procedures were performed at 4°C [Citation15].
Sephadex G-200 gel filtration chromatography
Dried Sephadex G-200 (2 g) was used for a 165 mL column (2 × 50 cm) bed volume. The gel was soaked in distilled water at 90°C for 5 h. After removal of the air in the gel, it was loaded onto the column and the flow rate adjusted to 15 mL/h by means of a peristaltic pump. Then the column was equilibrated with 50 mM Tris-HCl +50 mM KCl buffer, pH 7.0, until the final absorbance difference became 0 at 280 nm. The dialyzed sample was mixed with 5% glycerol. The final sample was loaded onto the column and elutions were collected in 2 mL aliquots. In each fraction, enzyme activity was determined at 340 nm. Active fractions were collected and stored at − 20°C for testing enzyme purity by electrophoresis [Citation15].
Protein determination
The protein content in all samples was quantified spectrophotometrically at 595 nm according to Bradford's method [Citation20], using bovine serum albumin as standard.
Sds Polyacrylamide Gel Electrophoresis (Sds-page)
The control of enzyme purity was carried out using Laemmli's procedure [Citation21] with 3% and 8% acrylamide concentrations for running and stacking gel, respectively. E. coli β-galactosidase (116,000), rabbit phosphorylase B (97,400), bovine albumin (66,000), chicken ovalbumin (45,000) and bovine carbonic anhydrase (29,000) were used as standards (Sigma: MW-SDS-200).
In vitro drug effects
In order to determine the effects of some drugs on human GR, concentrations of imipenem (0.01–0.08 mM), rifamycin (0.116–0.347 mM), sulfanylacetamide (0.36–0.72 mM), ceftazidime (0.915–3.66 mM), chloramphenicol (0.771–3.60 mM), seftriaxon (18.89–37.78 mM), vancomycin (3.45–10.35 mM), cefuroxime (4.896–31.824 mM), ornidazole (7.59–37.95 mM), were added to the reaction medium. The enzyme activity in the absence of drug was used as a control (100% activity). The IC50 values were obtained from activity (%) vs. drug concentration plots and regression analysis graphs were drawn by a statistical package (SPSS-for windows; version 10.0) (student t-test; n = 3).
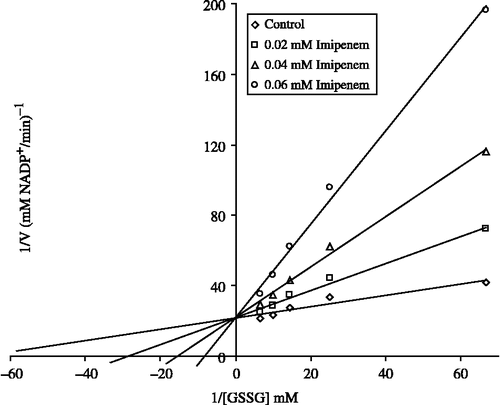
In order to determine Ki constants for the inhibitors, the substrate (GSSG) concentrations were 0.015, 0.04, 0.07, 0.10, and 0.15 mM. Inhibitors (drugs) solutions were added to the reaction medium, at 3 different fixed concentrations of inhibitors in 1 ml of total reaction volume. Lineweaver-Burk graphs [Citation22] were drawn by using 1/V vs. 1/[S] values and Ki constant were calculated from these graphs.
Results
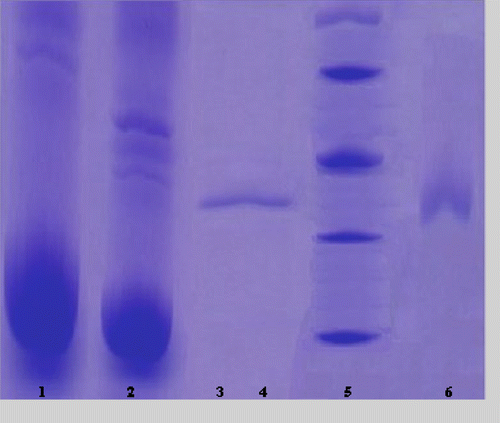
The purification of the enzyme led to a specific activity of 28 EU/mg protein, a yield of 29% and a purification coefficient of 2800 (). SDS polyacrylamide gel electrophoresis was performed after the purification of the enzyme, and the electrophoretic pattern was photographed ().
Table I. Purification of GR from human erythrocyte.
Figure 1 SDS-PAGE bands of GR (Lane 1: Hemolysate; Lane 2: Ammonium sulfate precipitation; Lane 3–4: Gel filtration chromatography; Lane 5: Standards: E.Coli βgalactosidase (116,000), rabbit phosphorylase B (97,400), bovine albumin (66,000), chicken ovalbumin (45,000), and bovine carbonic anhydrase (29,000); Lane 6: 2′, 5′-ADP Sepharose 4B affinity chromatography.
IC50 values of imipenem, rifamycin, sulfanylacetamide, ceftazidime, chloramphenicol, seftriaxon, vancomycin, cefuroxime and ornidazole were 0.030, 0.146, 0.59, 2.476, 2.36, 2.88, 4.83, 15.43 and 19.632 mM, respectively, and the Ki constants were 0.06 ± 0.01, 0.275 ± 0.10, 0.85 ± 0.05, 3.59 ± 0.51, 3.85 ± 0.40, 3.71 ± 0.60, 15.11 ± 2.50, 23.50 ± 2.94 and 28.49 ± 6.50 mM, respectively ().
Table II. Ki and IC50 values obtained from regression analysis graphs for GR in the presence of different drugs.
Discussion
Human GR from erythrocytes was purified in this study by hemolysate preparation, ammonium sulphate precipitation, 2′, 5′-ADP Sepharose 4B affinity chromatography and gel filtration chromatography. The purified preparation was characterized with a specific activity of 28 EU/mg protein, a yield of 29% and a purification coefficient of 2800. These figures tend to validate the procedure used in the study. SDS-PAGE showed the high purity of the enzyme.
The undesirable biological effects of oxidative agents, such as free radical and reactive oxygen species (ROS), are eliminated by enzymatic and nonenzymatic antioxidant defense systems. Enzymatic defense is provided by many enzyme systems such as glutathione reductase, glutathione peroxidase, glutathione S-transferase, superoxide dismutase, catalase, aldoketoreductase and DNA repair enzymes [Citation1,Citation23]. Particularly, GR is essential for the maintenance of cellular glutathione in its reduced form, which is highly nucleophilic for many reactive electrophiles [Citation24].
Nitro aromatic compounds can either be strong or weak inhibitors of erythrocyte GR [Citation26]. Similar results were obtained in different studies Citation16-18. As summarized by Asahi et al [Citation27], several enzymes have been shown to be inactivated or modified by NO. The modifications of these enzymes can be classified into two groups: NO binding to the iron of iron-cofactor-containing enzymes such as guanylyl cyclase, aconitase, cytochrome c oxidase and cyclooxygenase; and NO interacting with the SH group of enzymes containing a catalytically essential thiol such as GR [Citation27]. It was shown that aminoglycosides (streptomycin, gentamycin, netilmycin) generally activate erythrocyte GR Citation16-18 and we had similar results for amikacin.
However, to the best of our knowledge, the inhibitory effects of the drugs described here on erythrocyte GR have not previously been studied.
In order to show inhibitory effects, while the most suitable parameter is the Ki constant, some researchers use the IC50 value. Therefore, in this study, both the Ki and IC50 parameters of these drugs for GR were determined.
As shown in , the Ki values were 0.06 ± 0.01, 0.275 ± 0.10, 0.85 ± 0.05, 3.59 ± 0.51, 3.85 ± 0.40, 3.71 ± 0.60, 15.11 ± 2.50, 23.50 ± 2.94 and 28.49 ± 6.50 mM for imipenem, rifamycin, sulfanylacetamide, ceftazidime, chloramphenicol, seftriaxon, vancomycin, cefuroxime and ornidazole, respectively, and the corresponding IC50 values were 0.030, 0.146, 0.59, 2.476, 2.36, 2.88, 4.83, 15.43 and 19.632 mM, respectively. Ki values and IC50 values show that imipenem was the most potent inhibitor followed by rifamycin, sulfanylacetamide, ceftazidime, chloramphenicol, seftriaxon, vancomycin, cefuroxime and ornidazole, respectively.
In this investigation, these drugs showed highly inhibitory effects on the GR enzyme activity of human erythrocyte and the use of these drugs may have an undesirable effect on the enzyme, besides reducing fatty acid synthesis.
The plasma level of drugs used clinically is as follows; imipenem ∼0.315, rifamycin ∼0.0695, ceftazidime ∼0.366, chloramphenicol ∼0.617, seftriaxon∼0.302, vancomycin ∼0.345, cefuroxime ∼0.294 and ornidazole ∼0.455 mM [Citation25]. By taking into these concentrations into account, the inhibition data calculated from plots were found to be ∼>80%, ∼16.4%, ∼6.7%, ∼11.2%, 0%, ∼2.6%, and ∼1%, respectively. Here, only the IC50 and Lineweaver-Burk graphs for imipenem are shown ( and ). According to these data, if it is required to give imipenem and rifamycin to patients, their dosage should be very well controlled to decrease hemolytic and other side effects.
References
- Meisler A, Anderson ME. Glutathione. Ann Rev Biochem 1983; 52: 711
- Gul M, Kutay FZ, Temocin S, Hanninen O. Cellular and clinical imllications of glutathione. Indian J Exp Biol 2000; 38: 625
- Schirmer RH, Krauth-Siegel RL, Schulz GE. Glutathione reducase. 1989; 553, New York, John Wiley and Sons Press
- Akerlund B, Tynell E, Bratt G, Bielentein M, Lidman C. Nacetylcysteine treatment and the risk of toxic reactions to trimethoprimsulphamethoxazole in primary Pneumocystis carinii prophylaxis in HIVinfected patients. J Infect 1997; 35: 143
- Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann Neurol 1998; 44: 72
- Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: Regulation of glutathione synthesis and efflux. Diabetologia 1995; 38: 201
- Vijayalingam S, Parthiban A, Shanmugasundaram KR, Mohan V. Abnormal antioxidant status in impaired glucose tolerance and non-insulindependent diabetes mellitus. Diabet Med 1996; 13: 715
- Srivastava SK, Beutler E. Glutathione metabolism of the erythrocyte. The enzymic clevage of glutathione-haemoglobin preparations by glutathione reductase. Biochem J 1970; 119: 353
- Scott EM, Duncan IEW, Ekstrand V. Purification and properties of glutathione reductase of human erythrocytes. J Biol Chem 1963; 238: 3928
- Staal GEJ, Visser J, Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim Biophys Acta 1969; 185: 39
- Krohne-Ehrich G, Schirmer RH, Untucht-Grau R. Glutathione reductase of human erythrocytes; Isolation of the enzyme and sequence analaysis of the redox-active peptide. Eur J Biochem 1971; 80: 65
- Worthington DJ, Rosemeyer MA. Human glutathione reductase: Purification of the crystalline enzyme from erythrocytes. Eur J Biochem 1974; 48: 167
- Boggaram V, Brobjer T, Larson K, Mannervik B. Purification of glutathione reductase from porcine erythrocytes by the use of affinity chromatography on 2′,5′-ADP-Sepharose 4B and crystallization of the enzyme. Anal Biochem 1979; 98: 335
- Thıeme RR, Paı EF, Schırmer RH, Schulz GE. 3-dimensional structure of glutathione-reductase at 2 a resolution. J Biol 1981; 152: 763
- Erat M, Ciftci M. Effect of melatonin on enzyme activites of glutathione reductase from human erythrocytes in vitro and from rat erythrocytes in vivo. Eur J Pharmacol 2006; 537: 59
- Erat M, Sakiroglu H, Ciftci M. Effects of some antibiotics on glutathione reductase activities from human erythrocytes in vitro and from rat erythrocytes in vivo. J Enz Inhıb Med Chem 2005; 20: 69
- Erat M, Sakiroglu H, Ciftci M. Effects of some antibiotics on glutathione reductase from bovine erythrocytes. Vet Med 2003; 48: 305
- Erat M, Ciftci M. In vitro effects of some antibiotics on glutathione reductase from sheep liver. J Enz Inhıb Med Chem 2003; 18: 545
- Beutler E, Red Cell Metabolism. A manual of biochemical methods. Grune and Stratton Inc., Orlando 1984; 134
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934; 56: 658–666
- Ulusu G, Erat M, Ciftci M, Sakiroglu H, Bakan E. Purification and characterization of glutathione reductase from sheep liver. Turkish Journal of Veterinary & Animal Science 2005; 29: 1109
- Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 1975; 250: 5475
- Kayaalp SO. Rasyonal tedavi yönünden tıbbi farmakoloji. Hacettepe-Tas Yayıncılık (Turkish), Ankara 2002
- Grellier P, Sarlauskas J, Anusevicius Z, Maroziene A, Houee-Levin C, Schrevel J, Cenas N. Antiplasmodial activity of nitroaromatic and quinoidal compounds: Redox potential vs inhibition of erythrocyte glutathione reductase. Arch Biochem Biophys 2001; 393: 199
- Asahi M, Fujii J, Suzuki K, Seo HG, Kuzuya T, Hori M, Tada M, Fujii S, Taniguchi N. Inactivation of glutathione peroxidase by nitric oxide. J Biol Chem 1995; 270: 21035

![Figure 2 Activity % vs [Imipenem] regression analysis graphs for human erythrocytes GR in the presence of 5 different imipenem concentrations.](/cms/asset/c399f2fb-33a6-487a-b75a-8c6265d23113/ienz_a_234152_f0002_b.gif)