Abstract
Six AChE monooxime-monocarbamoyl reactivators with an (E)-but-2-ene linker were synthesized using modification of currently known synthetic pathways. Their potency to reactivate AChE inhibited by the nerve agent tabun and insecticide paraoxon was tested in vitro. The reactivation efficacies of pralidoxime, HI-6, obidoxime, K048, K075 and the newly prepared reactivators were compared. According to the results obtained, one reactivator seems to be promising against tabun-inhibited AChE and two reactivators against paraoxon-inhibited AChE. The best results were obtained for bisquaternary substances with at least one oxime group in position four.
Introduction
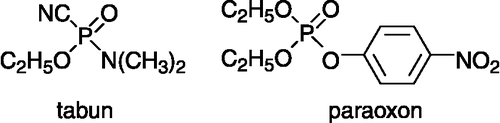
The enzyme acetylcholinesterase (AChE, EC 3.1.1.7) plays a very important role in the human body. It controls cholinergic transmission by decomposition of the neuromediator acetylcholine. The blockade of its physiological function by various inhibitors could be used for treatment of Alzheimer disease (competitive inhibitors) or misused for military or terrorist activity (irreversible inhibitors) Citation1-5. The well known irreversible inhibitors are organophosphorus compounds (OPC) [Citation6]; thio- or oxo-derivates of phosphonic and phosphoric acid [Citation6]. They are used extensively in agriculture as pesticides (e.g. parathion, chlorpyrifos, diazinon), for industrial purposes (e.g. tributylphosphate), and were also misused as nerve agents (e.g. sarin, soman, tabun, VX) in local conflicts and by terrorists () Citation7-10.
The mechanism of the enzyme's inhibition by OPC consists in covalent binding on the serine hydroxyl in the cavity of AChE [Citation6]. The cavity contains one catalytic (acylation, A) site at the bottom and one peripheral site (P) at the lip of the cavity Citation11-12. The A-site contains the catalytic triad (for human AChE, S203, E334 and H447) which together with W86 is responsible for binding of the trimethylammonium group of acetylcholine as acyl transfer to Ser203 is initiated [Citation13]. The P-site involves other residues including W286 [Citation14]. The ligands bounded to the P-site can affect the A-site by steric blockade or allosteric activation. This narrow 20 Å deep gorge was studied in detail by ligand binding and crystallographic studies for various species Citation13-16.
In addition, structural changes occur during OPC-inhibition [Citation16]. The OPC-AChE conjugate may undergo further intramolecular modifications called “aging” that involves dealkylation or deamidation [Citation17]. The aged AChE is inhibited completely and can not be reactivated. One of the most resistant inhibitions is caused by the nerve agent tabun (GA) [Citation18]. Although the structural basis for resistance of tabun conjugates is unknown, the crystal structures of murine AChE showed that non-aged tabun conjugate induces structural changes in H447 and its hydrogen bonds [Citation16,Citation19]. Moreover, the conformational change of the P338 position partially closes the narrow AChE gorge [Citation16,Citation19]. After an aging reaction, the phosphoramidoyl group of GA is replaced by a molecule of water and the rest of GA molecule is coordinated in the enzyme's cavity [Citation16]. Therefore GA belongs to the worst reactivatable nerve agents.
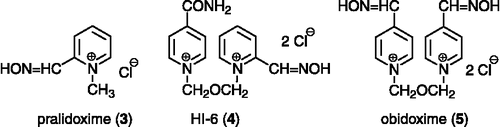
The reactivation process consists in cleavage of the covalent bond OPC-AChE by a nucleophilic group (oximate anion) which restores the activity of AChE [Citation1]. The reactivators of AChE used are oximes e.g. pralidoxime, obidoxime, HI-6 () [Citation2,Citation6]. Nevertheless, every type of OPC needs a specific structure of its AChE reactivator due to the huge variety of substituents on the central phosphorus atom in the molecule of the OPC [Citation6]. There is not a broad spectrum reactivator after more than fifty years of investigations Citation17-18 so that, the development and selection of new effective reactivators as antidotes to OPC inhibited-AChE is very important.
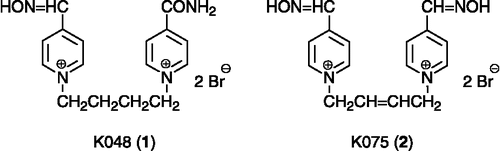
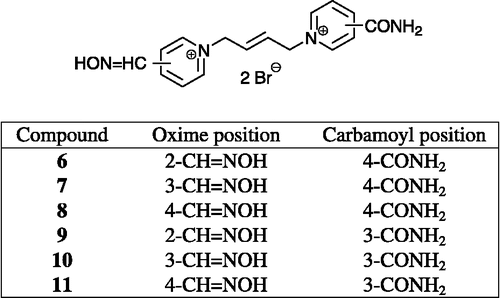
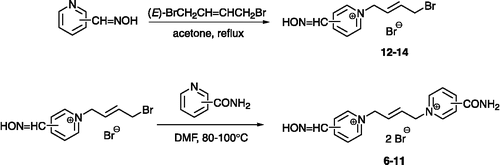
Several potent substances against GA-inhibited AChE were investigated recently in our group – K048 (1) and K075 (2) () Citation20-22. In order to combine these two molecules, new potential reactivators were designed. The carbamoyl group replaced one oxime group in the molecule of K075. The (E)-but-2-ene linker connecting the pyridinium rings is also in compound K075, which is slightly shorter than the saturated one, supplying the butane connecting chain in the molecule of K048. Six compounds (6–11) were prepared using conventional synthetic procedures; five of them (7–11) have not been previously described in the literature (). Firstly, the monoquaternary salts (12–14) were synthesized using an excess of five equivalent of (E)-1,4-dibromobut-2-ene in acetone, where bi-products occur only in minor yields (Scheme ). The mono-salts were purified by recrystallization from acetonitrile (MeCN), where the bis-salts were almost insoluble. Secondly, the bisquaternary substances were formed in DMF using an excess of the corresponding carbamoylpyridine (Scheme ) Citation23-26. Unfortunately, derivatives of 2-carbamoylpyridine could not be prepared although synthesis was examined both via the hydroxyiminomethylpyridine and carbamoylpyridine direction. The only previously known oxime BI-6 (6) was prepared by a novel approach and, moreover, NMR and MS analysis were determined, since the foregoing literature data were not available [Citation26]. The synthesized compounds were tested in vitro on tabun (GA)-and paraoxon-inhibited AChE and compared to known oximes (pralidoxime, obidoxime, HI-6) and promising oximes against GA (K048, K075) Citation20-22.
Materials and methods
Chemistry
Preparation of quaternary salts
(A) Preparation of monoquaternary salts – A solution of the hydroxyiminomethylpyridine (2.0 g, 16.4 mmol) and (E)-1,4-dibromobut-2-ene (17.51 g, 81.9 mmol) in acetone (60 mL) was stirred at reflux. The reaction mixture was cooled to room temperature and the crystalline crude product collected by filtration, washed with acetone (3 × 20 mL) and recrystallized from MeCN (12–14). (B) Preparation of bisquaternary salts – A solution of the monoquaternary salt (0.50 g, 1.5 mmol) and carbamoylpyridine (0.38 g, 3.0 mmol) in DMF (10 mL) was stirred at 80–100°C. The reaction mixture was cooled to room temperature and portioned with acetone (50 mL); the crystalline crude product was collected by filtration, washed with acetone (3 × 20 mL) and recrystallized from MeCN (6–11).
(E)-1-(4-carbamoylpyridinium)-4-(2-hydroxyiminomethylpyridinium)-but-2-ene dibromide (6). Prepared by method B via (12)
The reaction mixture was stirred at 80°C and stopped after 3 h. Yield 0.52 g (76%), TLC Rf 0.15, m.p. 197–199°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.22 (d, 2H, J = 6.0 Hz, PyrH), 9.13 (d, 1H, J = 6.0 Hz, PyrH), 8.75 (s, 1H, –CONH2), 8.68 (s, 1H, –CH = NOH), 8.65-8.56 (m, 1H, PyrH), 8.52-8.38 (m, 3H, PyrH), 8.32 (s, 1H, –CONH2), 8.21-8.12 (m, 1H, PyrH), 6.40-6.26 (m, 1H, –CH = ), 6.05-5.91 (m, 1H, –CH = ), 5.60 (d, 2H, J = 4.7 Hz, –CH2-), 5.39 (d, 2H, J = 6.3 Hz, –CH2-). 13C NMR (75 MHz, DMSO d6): δ (ppm) 167.15, 146.22, 145.24, 144.91, 143.57, 143.02, 134.39, 130.98, 130.44, 129.09, 127.16, 62.60. EA: Calculated 41.95% C, 3.96% H, 12.23% N; Found 41.78% C, 4.17% H, 12.15% N. ESI-MS: m/z 149.1 [M]2 + (calculated for [C8H9N2O]2 + 149.17).
(E)-1-(4-carbamoylpyridinium)-4-(3-hydroxyiminomethylpyridinium)-but-2-ene dibromide (7). Prepared by method B via (13)
The reaction mixture was stirred at 100°C and stopped after 3 h. Yield 0.58 g (85%), TLC Rf 0.15, m.p. 233–235°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.37.-9.28 (m, 3H, PyrH), 9.11 (d, 1H, J = 6.0 Hz, PyrH), 8.82-8.72 (m, 2H, PyrH + –CONH2), 8.49 (d, 2H, J = 6.0 Hz, PyrH), 8.41 (s, 1H, –CH = NOH), 8.31 (s, 1H, –CONH2), 8.25-8.17 (m, 1H, PyrH), 6.31-6.23 (m, 2H, –CH = ), 5.50-5.39 (m, 4H, -CH2-). 13C NMR (75 MHz, DMSO d6): δ (ppm) 163.18, 148.35, 145.89, 144.50, 143.19, 142.57, 141.79, 133.37, 130.13, 130.08, 128.17, 125.87, 61.00, 60.79. EA: Calculated 41.95% C, 3.96% H, 12.23% N; Found 41.85% C, 4.09% H, 12.19% N. ESI-MS: m/z 149.1 [M]2 + (calculated for [C8H9N2O]2 + 149.17).
(E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene dibromide (8). Prepared by method B via (14)
The reaction mixture was stirred at 100°C and stopped after 2 h. Yield 0.56 g (82%), TLC Rf 0.15, m.p. 240–241°C. 1H NMR (300 MHz, D2O d6): δ (ppm) 9.06 (d, 2H, J = 6.0 Hz, PyrH), 8.84 (d, 2H, J = 6.0 Hz, PyrH), 8.44-8.36 (m, 3H, PyrH + –CH = NOH), 8.23 (d, 2H, J = 6.0 Hz, PyrH), 6.43-6.25 (m, 2H, –CH = ), 5.43 (d, 2H, J = 4.8 Hz, –CH2-), 5.34 (d, 2H, J = 4.8 Hz, –CH2-). 13C NMR (75 MHz, DMSO d6): δ (ppm) 149.81, 149.60, 146.77, 146.21, 146.19, 145.18, 131.28, 130.09, 127.17, 125.58, 62.63, 61.90. EA: Calculated 41.95% C, 3.96% H, 12.23% N; Found 41.38% C, 4.04% H, 12.09% N. ESI-MS: m/z 149.1 [M]2 + (calculated for [C8H9N2O]2 + 149.17).
(E)-1-(3-carbamoylpyridinium)-4-(2-hydroxyiminomethylpyridinium)-but-2-ene dibromide (9). Prepared by method B via (12)
The reaction mixture was stirred at 100°C and stopped after 2 h. Yield 0.56 g (82%), TLC Rf 0.15, m.p. 240–241°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.52 (s, 1H, PyrH), 9.17 (d, 1H, J = 6.0 Hz, PyrH), 9.12 (d, 1H, J = 6.0 Hz, PyrH), 9.00 (d, 1H, J = 7.8 Hz, PyrH), 8.70-8.56 (m, 3H, PyrH + –CH = NOH + -CONH2), 8.42 (d, 1H, J = 8.1 Hz, PyrH), 8.33-8.26 (m, 1H, PyrH), 8.24-8.12 (m, 2H, PyrH + –CONH2), 6.42-6.28 (m, 1H, –CH = ), 6.10-5.94 (m, 1H, –CH = ), 5.56 (d, 2H, J = 4.8 Hz, –CH2-), 5.39 (d, 2H, J = 6.3 Hz, –CH2-). 13C NMR (75 MHz, DMSO d6): δ (ppm) 162.61, 147.12, 146.19, 146.02, 145.72, 144.96, 143.73, 141.39, 133.57, 131.47, 127.73, 125.66, 61.07, 58.20. EA: Calculated 41.95% C, 3.96% H, 12.23% N; Found 41.70% C, 4.12% H, 12.05% N. ESI-MS: m/z 149.1 [M]2 + (calculated for [C8H9N2O]2 + 149.17).
(E)-1-(3-carbamoylpyridinium)-4-(3-hydroxyiminomethylpyridinium)-but-2-ene dibromide (10). Prepared by method B via (13)
The reaction mixture was stirred at 100°C and stopped after 3 h. Yield 0.59 g (87%), TLC Rf 0.15, m.p. 223–224°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.56 (s, 1H, PyrH), 9.33 (s, 1H, PyrH), 9.27 (d, 1H, J = 6.0 Hz, PyrH), 9.09 (d, 1H, J = 6.0 Hz, PyrH), 9.02 (d, 1H, J = 8.1 Hz, PyrH), 8.77 (d, 1H, J = 8.1 Hz, PyrH), 8.66 (s, 1H, –CONH2), 8.39 (s, 1H, –CH = NOH), 8.36-8.28 (m, 1H, PyrH), 8.25-8.16 (m, 2H, PyrH), 6.33-6.25 (m, 2H, –CH = ), 5.49-5.38 (m, 4H, –CH2-). 13C NMR (75 MHz, DMSO d6): δ (ppm) 162.66, 146.47, 144.98, 144.50, 143.76, 143.19, 142.56, 141.80, 133.59, 133.38, 130.21, 130.11, 128.16, 127.90, 61.08. EA: calculated 41.95% C, 3.96% H, 12.23% N; Found 41.70% C, 4.12% H, 12.18% N. ESI-MS: m/z 149.1 [M]2 + (calculated for [C8H9N2O]2 + 149.17).
(E)-1-(3-carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene dibromide (11). Prepared by method B via (14)
The reaction mixture was stirred at 100°C and stopped after 3 h. Yield 0.58 g (85%), TLC Rf 0.15, m.p. 222‐224°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.56 (s, 1H, PyrH), 9.26 (d, 1H, J = 6.0 Hz, PyrH), 9.07 (d, 2H, J = 6.0 Hz, PyrH), 9.02 (d, 1H, J = 8.1 Hz, PyrH), 8.67 (s, 1H, –CONH2), 8.46 (s, 1H, –CH = NOH), 8.35-8.24 (m, 3H, PyrH), 8.21 (s, 1H, –CONH2), 6.30-6.23 (m, 2H, –CH = ), 5.44 (d, 2H, J = 4.8 Hz, –CH2-), 5.36 (d, 2H, J = 4.4 Hz, –CH2-). 13C NMR (75 MHz, DMSO d6): δ (ppm) 162.66, 148.66, 146.47, 145.13, 145.04, 143.74, 133.60, 130.54, 129.70, 127.91, 124.02, 61.08, 60.29. EA: Calculated 41.95% C, 3.96% H, 12.23% N; Found 41.66% C, 4.07% H, 12.12% N. ESI-MS: m/z 149.1 [M]2 + (calculated for [C8H9N2O]2 + 149.17).
1-(4-Bromobut-2-enyl)-2-hydroxyiminomethylpyridinium bromide (12) Consistent with literature data [Citation24]
1-(4-Bromobut-2-enyl)-3-hydroxyiminomethylpyridinium bromide (13) Prepared by method A
The reaction mixture was stopped after 1.5 h. Yield 4.62 g (84%), TLC Rf 0.60, m.p. 107–111°C. 1H NMR (300 MHz, DMSO d6): δ (ppm) 9.29 (s, 1H, PyrH), 9.06 (d, 1H, J = 6.0 Hz, PyrH), 8.75 (d, 1H, J = 8.1 Hz, PyrH), 8.38 (s, 1H, -CH = NOH), 8.24-8.18 (m, 1H, PyrH), 6.26-6.15 (m, 2H, -CH = ), 5.39 (d, 2H, J = 5.5 Hz, -CH2-N), 4.18 (d, 2H, J = 6.2 Hz, -CH2-Br). 13C NMR (75 MHz, DMSO d6): δ (ppm) 144.19, 143.23, 134.07, 133.48, 128.26, 127.48, 60.99, 32.01. EA: Calculated 35.74% C, 3.60% H, 8.34% N; Found 35.36% C, 3.81% H, 8.15% N. ESI-MS: m/z 254.9 [M+]+ (calculated for [C10H12BrN2O+]+255.01).
1-(4-Bromobut-2-enyl)-4-hydroxyiminomethylpyridinium bromide (14) Consistent with literature data [Citation24]
Biochemistry
In vitro testing of synthesized oximes involved a standard collection of experimental procedures. The 10% rat brain homogenate was used as a source of AChE. The brain homogenate (0.5 mL) was mixed with 20 μL of an isopropanol solution of GA (O-ethyl-N,N-dimethylphosphoramidocyanidate, obtained from the Military facility Brno, 95% purity) or paraoxon (O,O-diethyl-O-(4-nitrophenyl)phosphate, analytical standard 99.2% from Sigma-Aldrich) and distilled water (0.5 mL) to achieve 95% inhibition of AChE. The mixture was incubated at 25°C for 30 min. 2.5 mL of sodium chloride (3 M) solution were added to the mixture and distilled water added to a volume of 23 mL. Finally, 2 mL of a solution of acetylcholine iodide (0.02 M) was added. The enzyme activity (analyzed by potentiometric titration of decomposed acetylcholine iodide) was measured at pH 7.6 and 25°C on an autotitrator RTS 822 (Radiometer, Denmark). The same procedure was repeated with enzyme further subjected to 10 min incubation with an aqueous solution of reactivator (0.2 mL of 10− 2 M or 10− 4 M reactivator solution), which replaced 0.2 mL of water. Activities of intact AChE (a0), inhibited AChE (ai) and reactivated AChE (ar) were deduced from the consumption of NaOH solution (0.01 M) with time. The percentage of reactivation (%) was calculated from the measured data according to the formula:
The whole method is described in detail in the work of Kuca and Cabal [Citation27]. Pralidoxime, HI-6 and obidoxime of HPLC purity, previously synthesized in our laboratory were used as references. Obtained data are summarized in .
Table I. Reactivation potencies of tested oximes.
Results and discussion
As was previously stated, it is extraordinary difficult to reactivate GA-inhibited AChE. Our results with known reactivators (3–5) confirmed this fact in full Citation20-22. On the other hand, the recently developed reactivators (1–2) showed promising activity. In addition, there are two reactivators (8, 11), in the synthesized compounds, which had some ability against GA-inhibited AChE, although poor. Compared to the known or recently developed compounds, the (8) exceeds all of these at both concentrations used. Moreover, the concentration more suitable for in vivo experiments is limited to 10− 4 M [Citation28]. Compound (8) is almost able to match with the best of the previously published oximes (2) at the concentration one fold lower [Citation22].
Secondly, the reactivators developed for treatment of intoxication with nerve agents are not suitable for pesticide poisoning Citation29-30. The obtained results for the known oximes (3–4) showed that their ability to reactivate paraoxon-inhibited AChE is poor, especially at 10− 5 M concentration. The exception is made by obidoxime (5), which had promising activity; otherwise, the higher toxicity of obidoxime compared to HI-6 is well known [Citation31]. Furthermore, the oxime K075 (2) exceeds all other published reactivators in the reactivation of paraoxon-inhibited AChE at 10− 5 M concentration. The novel compounds also showed promising activity. Three of them (6, 8, 11) exceeded pralidoxime and HI-6 at a concentration 10− 3 M. Moreover, two oximes (6, 11) reached or exceeded the activity of obidoxime at a concentration applicable in vivo. Finally, the oxime 11 surpassed all tested compounds in reactivation of paraoxon-inhibited AChE.
Consequently, the structure-activity relationship appropriate for reactivation of tabun- and paraoxon-inhibited AChE should be considered [Citation32]. Generally, five structural factors influence the reactivation ability of an agent – the presence, position and number of oxime groups, presence of quaternary nitrogen and structure of connecting linker in bispyridinium compounds [Citation32].
The presence of oxime is essential for all reactivators [Citation2]. The position of oxime determines which type of OPC will be better accessible for reactivation Citation18-19Citation33–34. In the event of GA, the hydroxyiminomethyl (oxime) in position-four is preferred (1–2, 5, 8, 11) Citation20-22. For paraoxon-inhibited AChE, position-four of the oxime group (2, 11) also gave better reactivation ability although the differences are not so remarkable (5 in contrast to 6) [Citation25]. Our results showed that the number of oximes present in the reactivator molecule is not a limiting factor for higher reactivation efficacy. The second oxime group can be replaced by a carbamoyl group without decrease in reactivating activity both for GA and paraoxon (2 in contrast to 1; 2 in contrast to 8; 2 in contrast to 11). The quaternary compounds, monoquaternary and even better bisquaternary, have usually increased affinity to AChE than the non-quaternary reactivators as was previously described [Citation31,Citation35]. The length and constitution of the connecting linker also plays an important role in the reactivation process Citation36-37. The optimal length of the connecting bridge lies between three (5) and four (1) carbon atoms (or equivalents) where the but-2-ene connecting linker (2, 6–11) is satisfactory due to the double bond [Citation38]. Moreover, the double bond enhances the rigidity of the linker by dislocation of free rotation of one bond in this linker. Additionally, the application of the but-2-ene connecting linker gives better results, compared to the relevant butane linker (1 in contrast to 2), at 10− 5 M concentration, which is more appropriate for human use.
In conclusion, a series of six reactivators have been prepared in satisfactory yield and purity. Their ability to reactivate GA-and paraoxon-inhibited AChE was measured in vitro. One compound exceeded all the reference compounds against tabun-inhibited AChE. Two compounds were found to be promising against paraoxon-inhibited AChE at concentrations accessible after administration in vivo. Pralidoxime and HI-6 were found to be unsuitable for use against pesticide inhibition of AChE. The reactivation potency of these compounds depends on structural factors such as the position of the functional oxime group on the pyridinium ring, presence of quaternary nitrogen and the constitution of the linking chain.
The authors express their appreciation to Mrs. M. Hrabinova and Ms. P. Hanusova for their technical assistance. The work was supported by a grant from the Grant Agency of Charles University No. 302/2005/B-CH/FaF and by a grant from the Ministry of Defence of Czech Republic No. FVZ0000501.
References
- Bajgar J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 2004, 38: 151–216
- Francotte P, Graindorge E, Boverie S, de Tullio P, Pirotte B. New trends in the design of drugs against Alzheimer's disease. Curr Med Chem 2004; 11: 1757–1778
- Patocka J, Kuca K, Dohnal V, Jun D. Chemical terrorism. Kontakt 2006; 4: 123–127, (in Czech).
- Satoh T, Hosokawa M. Organophosphates and their impact on the global environment. Neurotoxicology 2000; 21: 223–227
- Krivoy A, Layish I, Rotman E, Goldberg A, Yehezkelli Y. OP or not OP: The medical challenge at the chemical terrorism scene. Prehospital Disaster Med 2005; 20: 155–158
- Marrs TC. Organophosphate poisoning. Pharmacol Therapeut 1993; 58: 51–66
- Marklund A, Andersson B, Haglund P. Organophosphorus flame retardants and plasticizers in air from various indoor environments. J Environ Monit 2005; 7: 814–819
- Gupta R. In Toxicology of organophosphate & carbamate compounds. Elsevier Academic Press, London 2006; 1: 5–69
- Hodgson E, Rose RL. Organophosphorus chemicals: Potent inhibitors of the human metabolism of steroid hormones and xenobiotics. Drug Metab Rev 2006; 38: 149–162
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta 2006; 366: 1–13
- Saxena A, Fedorko JM, Vinayaka CR, Medhekar R, Radic Z, Taylor P, Lockridge O, Doctor BP. Aromatic amino-acid residues at the active and peripheral anionic sites control the binding of E2020 (Aricept) to cholinesterases. Eur J Biochem 2003; 270: 4447–4458
- Rosenberry TL, Johnson JL, Cusack B, Thomas JL, Emani S, Venkatasubban KS. Interactions between the peripheral site and the acylation site in acetylcholinesterase. Chem-Biol Inter 2005; 157–158: 181–189
- Radic Z, Manetsch R, Krasinski A, Raushel J, Yamauchi J, Garcia C, Kolb H, Sharpless KB, Taylor P. Molecular basis of interactions of cholinesterases with tight binding inhibitors. Chem-Biol Inter 2005; 157–158: 133–141
- Axelsen PH, Harel M, Silman I, Sussman J. Structure and dynamics of the active site gorge of acetylcholinesterase: Synergistic use of molecular dynamics simulation and X-ray crystallography. Protein Sci 1994; 3: 188–197
- Ashani Y, Radic Z, Tsilgeny I, Vellom DC, Pickering NA, Quinn DM, Doctor BP, Taylor P. Amino acid residues controlling reactivation of organophosphonyl conjugates of acetylcholinesterase by mono- and bisquaternary oximes. J Biol Chem 1995; 270: 6370–6380
- Ekstrom F, Akfur C, Tunemalm AK, Lundberg S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry 2006; 45: 74–81
- Patocka J, Kuca K, Jun D. Acetylcholinesterase and butyrylcholinesterase-important enzymes of human body. Acta Medica 2004; 47: 215–228
- Cabal J, Kuca K, Kassa J. Specification of the structure of oximes able to reactivate tabun-inhibited acetylcholinesterase. Basic Clin Pharmacol Toxicol 2004; 95: 81–86
- Ekstrom F, Pang YP, Boman M, Artursson E, Akfur C, Borjegren S. Crystal structures of acetylcholinesterase in complex with HI-6, Ortho-7 and obidoxime: Structural basis for differences in the ability to reactivate tabun conjugates. Biochem Pharmacol 2006; 72: 597–607
- Kuca K, Bielavsky J, Cabal J, Kassa J. Synthesis of a new reactivator of tabun-inhibited acetylcholinesterase. Bioorg Med Chem Lett 2003; 13: 3545–3547
- Kuca K, Kassa J. A comparison of the ability of a new bispyridinium oxime–1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium)butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vitro methods. J Enz Inhib Med Chem 2003; 18: 529–535
- Kuca K, Cabal J, Musilek K, Jun D, Bajgar J. Effective bisquaternary reactivators of tabun-inhibited AChE. J Appl Toxicol 2005; 25: 491–495
- Musilek K, Kuca K, Jun D, Dohnal V, Dolezal M. Synthesis of a novel series of bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. J Enz Inhib Med Chem 2005; 20: 409–415
- Musilek K, Kuca K, Jun D, Dohnal V, Dolezal M. Synthesis of the novel series of bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. Bioorg Med Chem Lett 2006; 16: 622–627
- Musilek K, Holas O, Kuca K, Jun D, Dohnal V, Dolezal M. Synthesis of asymmetrical bispyridinium compounds bearing cyano-moiety and evaluation of their reactivation activity against tabun and paraoxon-inhibited acetylcholinesterase. Bioorg Med Chem Lett 2006; 16: 5673–5676
- Bielavsky J, Kassa J, Elsnerova I, Dejmek L. Cholinesterase reactivators derived from pyridine-2-carboxaldehyde. Coll Czech Chem C 1998; 63: 199–204
- Kuca K, Cabal J. Evaluation of newly synthesized reactivators of the brain cholinesterase inhibited by sarin nerve agent. Toxicol Mech Method 2005; 15: 247–252
- Tattersall JE. Ion channel blockade by oximes and recovery of diaphragm muscle from soman poisoning in vitro. Br J Pharmacol 1993; 108: 1006–1015
- Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol 2004; 68: 2237–2248
- Racakova V, Jun D, Opletalova V, Kuca K. Reactivation of acetycholinesterase inhibited by the pesticide chlorpyrifos. J Appl Biomed 2006; 4: 147–151
- Bartosova L, Kuca K, Kunesova G, Jun D. The acute toxicity of acetylcholinesterase reactivators in mice in relation to their structure. Neurotox Res 2006; 9: 291–296
- Kuca K, Jun D, Musilek K. Structural requirements of acetylcholinesterase reactivators. Mini-Rev Med Chem 2006; 6: 269–277
- Hrabinova M, Musilek K, Jun D, Kuca K. New group of xylene linker-containing acetylcholinesterase reactivators as antidotes against the nerve agent cyclosarin. J Enz Inhib Med Chem 2006; 21: 515–519
- Kuca K, Cabal J, Jun D, Bajgar J, Hrabinova M. Potency of new structurally different oximes to reactivate cyclosarin-inhibited human brain acetylcholinesterases. J Enz Inhib Med Chem 2006; 21: 663–666
- Picha J, Kuca K, Kivala M, Kohout M, Cabal J, Liska F. A new group of monoquaternary reactivators of acetylcholinesterase inhibited by nerve agents. J Enz Inhib Med Chem 2005; 20: 233–237
- Chennamaneni SR, Vobalaboina V, Garlapati A. Quaternary salts of 4,3′ and 4,4′ bis-pyridinium monooximes: Synthesis and biological activity. Bioorg Med Chem Lett 2005; 15: 3076–3080
- Srinivas Rao C, Venkateswarlu V, Achaiah G. Quaternary salts of 4,3′ and 4,4′ bis-pyridinium monooximes. Part 2: Synthesis and biological activity. Bioorg Med Chem Lett 2006; 16: 2134–2138
- Pang YP, Kollmeyer TM, Hong F, Lee JC, Hammond PI, Haugabouk SP, Brimijoin S. Rational design of alkylene-linked bis-pyridiniumaldoximes as improved acetylcholinesterase reactivators. Chem Biol 2003; 10: 491–502