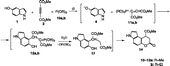Abstract
Angular pyrrolocoumarins were synthesized from the reaction of 4-hydroxyindole or 5-hydroxyindole with DMAD and PPh3 and were tested for anti-inflammatory and antioxidant activity. These compounds significantly inhibited the carrageenin-induced paw edema (60.5%–73.4%) and have important scavenging activity. Although their interaction with the free stable radical DPPH is not high, compound 9 is the most potent (73.4%) in the in vivo experiment. Compound 7 seems to be a potent LOX inhibitor. An attempt was made to correlate the biological results with their structural characteristics and physicochemical parameters.
Introduction
Coumarins constitute an important class of compounds with a wide range of biological properties. Citation1-3 In particular, furocoumarins are important as photochemotherapeutic agents that are used to treat a variety of skin diseases; [Citation4] they have been also found to exhibit antitumor, [Citation5] antioxidant [Citation3] and anti-inflammatory [Citation3] activities. Pyrrolocoumarins are nitrogenated isosteres of furocoumarins, which show photobiological and antitumor [Citation6] activities, cytotoxicity and HIV-integrase inhibition [Citation7] and they have been studied much less extensively.
The synthesis of pyrrolocoumarins is achieved by two main routes. In the first the formation of the pyrrole fragment is achieved from the reaction of aminocoumarins and benzoin, [Citation8] or the Fischer reaction of the corresponding hydrazones, Citation9-12 or from amine oxide rearrangement of the corresponding N-methyl-N-prop-2-ynylcoumarins, [Citation13] or from chloroacetylation of aminocoumarin and subsequent reduction [Citation14]. In the second route the pyranone fragment is introduced by Pechmann cyclization of hydroxyindole [Citation15] or by formylation of hydroxyindole and cyclization with acetic anhydride. [Citation16]
In the course of our continuing interest on the synthesis Citation17-20 of condensed coumarin derivatives with anti-inflammatory and antioxidant activities Citation21-23 including furocoumarins, [Citation18,Citation22] we have extended our research to the synthesis and biological evaluation of new fused pyrrolocoumarins as anti-inflammatory, antioxidant and possibly antithrombotic agents. Inflammation, as a complex phenomenon, is implicated in cardiovascular diseases and coumarin derivatives are used as antithrombotic agents.
Materials and methods
UV-Vis spectra were obtained on a Perkin-Elmer Lambda 20 double beam spectrophotometer and on a Hitachi U-2001 spectrophotometer. IR spectra were obtained with a Perkin-Elmer 1310 spectrophotometer as Nujol mulls. NMR spectra were recorded on a Bruker AM 300 (300 MHz and 75 MHz for 1H and 13C, respectively) using CDCl3 as solvent and TMS as an internal standard. Mass spectra were determined on a VG-250 spectrometer at 70 eV under Electron Impact (EI) conditions. Microanalyses were performed on a Perkin-Elmer 2400-II Element analyzer. Dichloromethane (DCM) was used as recrystalization solvent for the new compounds.
All the chemicals used were of analytical grade and commercially available. 5-Hydroxyindole, 1,1-diphenyl-2-picrylhydrazyl (DPPH), nordihydroguairetic acid (NDGA) were from the Aldrich Chemical Co. Milwaukee, WI, (USA). 4-Hydroxyindole was from Alfa Aesar GmbH & Co. Arachidonic Acid (AA), nicotinamido-adenine-dinucleotide (NADH), Nitrotetrazolium Blue (NBT), porcine heme, butylated hydroxytoluene (BHT), soybean lipoxygenase, linoleic acid sodium salt and indomethacin were from Sigma Chemical, Co. (St. Louis, MO, USA). N-methylphenazonium-methyl sulfate was from Fluka. Carrageenin, type K, is commercially available. Silica gel No 60, Merck A.G. was used for column chromatography.
SimplastinR Excel (Bio Merieux) (includes, thromboplastin reagent, aPTT reagent: cephaline – caoline) was used.
Synthesis
General procedure for the reactions of hydroxyindoles with DMAD and PPh3
Hydroxyindole [0.665 g (5 mmole)] was added to a solution of 1.31 g (5 mmole) triphenylphosphine (PPh3) in 20 mL dichloromethane (DCM) at 0°C. To the mixture, at that temperature, a solution of dimethylacetylenedicarboxylate (DMAD) [0.6 mL (5 mmole)] in DCM (20 mL) was added dropwise during 45 min. The orange mixture was then refluxed for 6 h. After cooling the mixture was evaporated and separated by column chromatography [ethyl acetate/hexane (1:1)] to give, after the elution of PPh3, the coumarin derivative (7 or 9).
Methyl 7-oxo-3,7-dihydropyrano[3,2-e]indole-9-carboxylate (7)
Yellow crystals [0.652 g (54% yield)]; m.p. 224–226°C; IR (Nujol, cm− 1): 3370, 1726, 1700; 1H-NMR (300 MHz, CDCl3) δ 4.09 (s, 3H), 6.55 (s, 2H), 7.24 (d, J = 8.8 Hz, 1H), 7.37 (t, J = 2.9 Hz, 1H), 7.62 (d, J = 8.8 Hz, 1H), 8.59 (brs, 1H); 13C-NMR (75.5 MHz, CDCl3) δ 53.6, 102.7, 108.9, 112.4, 114.6, 116.8, 120.5, 126.8, 146.1, 148.1, 156.6, 160.4, 165.4; MS m/z 243 (M+., 100), 212 (45), 211 (60), 184 (50), 156 (20), 155 (25), 128 (32), 101 (5) Anal. Calcd. for C13H9NO4 C, 64.18; H, 3.73; N, 5.76. Found: C, 64.33; H, 3.95; N, 5.65%.
Methyl 2-oxo-2,7-dihydropyrano[2,3-e]indole-4-carboxylate (9)
Yellow crystals [0.466 g (38% yield)]; m.p. 211–212°C; IR (Nujol, cm− 1): 3330, 1725, 1710; 1H-NMR (300 MHz, CDCl3) δ 4.01 (s, 3H), 6.79 (s, 1H), 6.97 (t, J = 2.9 Hz, 1H), 7.27 (d, J = 2.9 Hz, 1H), 7.34 (d, J = 8.8 Hz, 1H), 7.96 (d, J = 8.8 Hz, 1H), 8.53 (brs, 1H); 13C-NMR (75.5 MHz, CDCl3) δ 53.0, 101.3, 108.8, 111.1, 114.7, 119.9, 124.9, 128.1, 141.5, 149.8, 154.6, 161.0, 165.2; MS m/z 243 (M+., 100%), 215 (42), 184 (30), 156 (10), 128 (25), 101 (5). Anal. Calcd. for C13H9NO4 C, 64.18; H, 3.73; N, 5.76. Found: C, 64.05; H, 3.83; N, 5.62%.
General procedure for the reactions of 5-hydroxyindole with DMAD and P(OR)3
Trialkylphosphite (5 mmole) was added to a stirred solution of 5-hydroxyindole [0.665 g (5 mmole)] and DMAD [0.6 ml (5 mmole)] in DCM (50 mL) at room temperature and the yellow solution stirred at room temperature for 4 days. After evaporation of the solvent the resulting mixture was separated by column chromatography [ethyl acetate/hexane (1:1)] to give the indole derivative 14 (after the elution of phosphite).
Methyl 7-oxo-3,7,8,9-tetrahydropyrano[3,2-e]indole-9-carboxylate (14)
White crystals [0.413 g (34% yield) from P(OCH3)3, 0.623 g (51% yield) from P(OCH2CH3)3]; m.p. 181-183°C; IR (Nujol, cm− 1): 3410, 1754, 1726; 1H-NMR (300 MHz, CDCl3) δ 2.90 (dd, J1 = 6.6 Hz, J2 = 16.2 Hz, 1H), 3.20 (dd, J1 = 1.8 Hz, J2 = 16.2 Hz, 1H), 3.67 (s, 3H), 4.30 (dd, J1 = 1.8 Hz, J2 = 6.6 Hz, 1H), 6.67 (t, J = 3.0 Hz, 1H), 6.95 (d, J = 8.3 Hz, 1H), 7.33 (d, J = 3.0 Hz, 1H), 7.34 (d, J = 8.3 Hz, 1H), 8.43 (brs, 1H); 13C-NMR (75.5 MHz, CDCl3) δ 31.4, 39.3, 52.6, 101.1, 110.2, 112.1, 112.4, 126.3, 130.7, 136.1, 151.5, 167.0, 171.5; MS m/z 245 (M+, 50%), 186 (49), 185 (100), 158 (32), 157 (36), 142 (6), 130 (51), 102 (7). Anal. Calcd. for C13H11NO4 C, 63.66; H, 4.52; N, 5.71. Found: C, 63.70; H, 4.64; N, 5.63%.
Biological assay
In vivo
Inhibition of the carrageenin-induced oedema [Citation23]
Oedema was induced in the right hind paw of Fisher 344 rats (150–200 g) by the intradermal injection of 0.1 mL 2% carrageenin in water. Both sexes were used. Pregnant females were excluded. The animals, which have been bred in our laboratory, were housed under standard conditions and received a diet of commercial food pellets and water ad libitum during their maintenance but they were entirely fasted during the experimental period. Our studies were in accordance with recognised guidelines on animal experimentation (Guidelines for the care and use of laboratory animals published by the Greek Goverment 160/1991, based on EU regulations 86/609).
The tested compounds, 0.01 mmol/Kg body weight, were suspended in water with a few drops of Tween 80 and ground in a mortar before use and were given intraperitoneally simultaneously. The rats were euthanized 3.5 h after carrageenin injection. The difference between the weight of the injected and uninjected paws was calculated for each animal. The change in paw weight was compared with that in control animals (treated with water) and expressed as a percent inhibition of the carrageenin paw oedema (CPE %) values (). Indomethacin at 0.01 mmol/Kg (47% inhibition of the carrageenin paw oedema CPE) was administered as a standard drug for comparison reasons. Values CPE % are the mean from two different experiments with a standard error of the mean less than 10% (p < 0.01 compared with control values).
In vitro
Interaction of the tested compounds with 1,1-diphenyl-2-picryl-hydrazyl (DPPH) stable free radical [Citation24]
To a solution of DPPH (0.1 mM) in absolute ethanol an equal volume of the compounds (0.1 and 0.2 mM) dissolved in ethanol was added. Ethanol was used as a control solution. After 20 min at room temperature the absorbance was recorded at 517 nm. NDGA was used as an appropriate standard ().
Table I. Interaction % with DPPH (RA %); Competition % with DMSO for hydroxyl radical (HO√ %); % Superoxide radical scavenging activity (PMS %).
Soybean lipoxygenase inhibition [Citation24]
The tested compounds dissolved in DMSO (final concentration 0.1 mM) were incubated in room temperature with sodium linoleate (0.1 mM) and 0.15 mL of enzyme solution (1/9 × 104 w/v in saline). The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded and compared with the appropriate standard (caffeic acid 0.6 mM)
Non-enzymatic assay of superoxide radicals- measurement of superoxide radical scavenging activity [Citation24,Citation25]
The superoxide producing system was set up by mixing phenazine methosulfate (PMS), NADH and air–oxygen. The production of superoxide was estimated by the nitroblue tetrazolium method. The reaction mixture containing 3 μM PMS, 78 μM NADH, and 25 μM NBT in 19 μM phosphate buffer pH 7.4 was incubated for 2 min at room temperature and the absorption measured at 560 nm against a blank containing PMS. The tested compounds were preincubated for 2 min before adding NADH (). Caffeic acid was used as a standard (0.1 mM)
Competition of the tested compounds with DMSO for hydroxyl radicals [Citation24]
The hydroxyl radicals generated by the Fe3 + /ascorbic acid system, were detected by the determination of formaldehyde produced from the oxidation of DMSO. The reaction mixture contained EDTA (0.1 mM), Fe3 + (167 μM), DMSO (33 mM) in phosphate buffer (50 mM, pH 7.4), the tested compounds (concentration 0.01 mM and 0.1 mM) and ascorbic acid (10 mM). After 30 min of incubation (37o C) the reaction was stopped with CCl3COOH (17% w/v) (). Trolox was used as a control ().
Measurement of prothrombin time [Citation26]
50 μl of the examined compounds (7, 9, 14, warfarin and coumarin at final concentration 0.17 mM) and 50 μL of plasma from human healthy volunteers were reacted with 100 μL SimplastinR excel reagent containing rabbit thromboplastin and 100 μL calcium ions solution dissolved in buffer with stabilizers (CaCl2). The mixture (final volume 300 μL) was incubated at 37°C and the time of clot formation was measured ().
Measurement of activated partial thromboplastin time [Citation27]
50 μL of the tested compounds and 50 μL plasma from human healthy volunteers were incubated with 100 μL of cephaline – caoline at 37°C. After that 100 μL of CaCl2 (0.025 M) was added and the time was measured ().
In vitro / In vivo assays
Each experiment in vitro was performed at least in triplicate and the standard deviation of absorbance was less than 10%. Analysis of variance (ANOVA test) was used to assess significant differences. Differences at p < 0.05 were considered to be significant. In vivo statistical studies were done with student's T-test.
Results and discussion
Synthesis
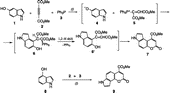
Synthesis was achieved in one step by a new pathway from hydroxyindoles and is based on the recently Citation28-29 reported formation of 4-methoxycarbonylcoumarin derivatives by the reaction of phenols with PPh3 and DMAD. The reaction studied and the products obtained are depicted in Schemes .
In a DCM solution of 5-hydroxyindole (1) was added dropwise at 0°C an equimolar amount of DMAD (2) in the presence of PPh3 (3) and the resulted orange solution was refluxed for 6 h to give compound 7 (54% yield) after separation of the complex reaction mixture by column chromatography with hexane/ethyl acetate (1:1) as eluent. The reaction of 4-hydroxyindole (8) with equimolar amounts 2 and 3 led to compound 9 (38% yield).
From the reaction of 1 with 2 in the presence of trimethylphosphite (10a) or triethylphosphite (10b) at room temperature over 4 days, compound 14 was isolated, after separation by column chromatography of the complex mixtures (34% or 51% yield, respectively). The 1H-NMR spectra of the products 7 and 14 showed both two doublets for the phenyl protons, in agreement with their angular structures.
The formation of compound 7 proceeds through the intermediates depicted in Scheme . An initial addition of the conjugate base, 4, to vinyltriphenylphosphonium salt, 5, leads, after a few transformations involving 1,2-H shift and abstraction of PPh3 from the intermediate ylide 6, to the formation of the substituted indolol 6‘, which by lactonization results in compound 7. A similar mechanism has been suggested for the formation of coumarins from the reactions between 2, 3 and phenols. [Citation28,Citation29] On the basis of analogous literature data for the reaction of 2-naphthol with phosphites in the presence of DMAD, [Citation30] it is reasonable to assume that the intermediate ylides 12a,b (Scheme ) are formed by attack of the carbon atom of the anion 4 to the initially formed adducts 11a,b. Hydrolysis of ylides 12a,b followed by lactonization of the resulted intermediate succinate 13 leads to the dihydro-compound 14.
Biological studies
The reported derivatives were tested for their antioxidant, anti-inflammatory and anticoagulant activities. Non-steroidal anti-inflammatory drugs (NSAIDs) have a broad spectrum of effects and it has been suggested that the variations in both efficacy and their tolerability are partly due to differences in their physicochemical properties, which determine their distribution in the body and their ability to pass through and to enter the interior of membranes. Citation31-32 In acute toxicity experiments, the in vivo examined compounds did not present toxic effects in doses up to 0.5 mmol/kg body.
To assess the anti-inflammatory activity of the pyrrolocoumarins the rat carrageenin-induced paw Oedema assay was employed as a model for acute inflammation. It detects compounds that are anti-inflammatory agents, as a result of inhibition of prostaglandin amplification. Edema was induced in the right hind paw of Fischer 344 rats (150–200 g) by the intradermal injection of 0.1 ml 2% carrageenin in water. The tested compounds 0.01 mmol/kg body weight, were dissolved in water and were given intraperitoneally simultaneously. Indomethacin was included as a reference drug. It is of interest that all the tested compounds possessed significant protection which ranged from 60.5 to 73.4%. The small differences among clogP values (1.24–1.60) do not support the view that lipophilicity could affect the biological responses (). The compounds concerned were further evaluated for inhibition of soybean lipoxygenase LOX by the UV absorbance based enzyme assay. [Citation24] While one may not extrapolate the quantitative results of this assay to the inhibition of mammalian 5-LOX, it has been shown that inhibition of plant LOX activity by NSAIDs is qualitatively similar to their inhibition of the rat mast cell LOX and may be used as a simple qualitative screen for such activity (). Examination of LOX % inhibition and/or IC50's values shows that compound 7 is the most active, while compounds 9 and 14 present moderate inhibitory activity on LOX. Most of the LOX inhibitors are antioxidants or free radical scavengers [Citation33] since lipoxygenation occurs via a carbon-centered radical. Several LOX inhibitors are excellent ligands for Fe+3. Many coumarinyl and pyrrolyl derivatives inhibit soybean lipoxygenase. This inhibition is related to their ability to reduce the iron species in the active site to the catalytically inactive ferrous form. [Citation34] Nowadays, antioxidants that exhibit DPPH radical scavenging activity are increasingly receiving attention. They have been reported to have interesting anticancer, anti-ageing and anti-inflammatory activities. DPPH is a stable free radical that can accept an electron or hydrogen radical and thus be converted into a stable, diamagnetic molecule. DPPH has an odd electron and so has a strong absorption band at 517 nm. All compounds were tested for their interaction with the stable free radical DPPH and NDGA was used as reference compound. This interaction indicates their radical scavenging activity in an iron free system and expresses their reducing activity. The tested compounds were found to have low activity at 0.1 mM. Not many changes were observed with time of reaction (compound 14 presents an increase). Compounds 7 and 14 showed higher interactions with an increase in concentration. Compound 14 presents the highest activity at 0.1 mM. and 0.2 mM. In general the results did not proceed in parallel with the increase of time and concentration (). The competition of compounds with DMSO for OH√ radicals [Citation24], generated by the Fe3 + /ascorbic acid system, expressed as the inhibition of formaldehyde production, was used for the evaluation of their hydroxyl radical scavenging activity. Compound 9 could not be tested at 0.1 mM due to solubility problems. Compounds 7, 9, 14 significantly inhibited the DMSO oxidation at 0.01 mM concentration. The inhibition was not significantly affected by an increase in concentration ().
Table II. Inhibition % LOX / IC50 values of soybean lipoxygenase; Inhibition % of induced carrageenin rat paw edema (CPE %) at 0.01 mmol/Kg body weight; Theoretically calculated lipophilicity values. [Citation35]
Non-enzymatic superoxide anion radicals were generated [Citation25] by mixing phenazine methosulfate (PMS), NADH and air –oxygen and the production of superoxide was estimated by the nitroblue tetrazolium method. Compounds 7 and 14 did not show any activity under the experimental conditions but 9 showed high scavenging activity.
Quick et al [Citation26] introduced a prothrombin time (PT) assay as a screening test for factors VII, X, V and II which allows an accurate evaluation of the capacity of the extrinsic pathway; whereas the aPTT (partial activated thromboplastin time assay) is used to evaluate the efficacy to inhibit the intrinsic pathway of coagulation. The method consists in measuring the time up to clot formation after adding the “PR reagent” (a combination of tissue thromboplastin and Ca+2). [Citation27] All the tested compounds showed medium PT values whereas the aPTT values compared to the control were found to be significant. Compounds 7 and 14 presented comparable activities in both tests. Coumarin was also tested under the same experimental conditions and the results are given in . Since all these derivatives contain this functional structure, the biological results should be correlated with this.
Table III. PT and aPTT values (sec) induced by the tested compounds
The above compounds constitute an interesting template for the evaluation of new synthetic lipoxygenase inhibitors and may be helpful in the design of new therapeutic tools against inflammation. From our results, it can be concluded, that lipophilicity is not the main property responsible for the anti-inflammatory/antioxidant activity of the investigated coumarins. The in vivo antiinflammatory activity of the synthesized compounds seems to be related with their high HO√ scavenging ability and their good LOX inhibitory activity
For the C5-C6 fused derivatives, the absence of the double bond between C3-C4 is correlated with a decrease in the in vivo biological responses (compound 7, 67.8% and 14, 60.5%). The angular derivative 9 (fused at C7-C8) is more potent in vivo compared to compound 7. In general, stereochemistry of the molecules seems to affect their biological activities.
Acknowledgements
The authors gratefully acknowledge support of this work through the program PYTHAGORAS II of EPEAEK II (MIS: 97436/073). We would like also to thank Dr C. Hansch and Biobyte Corp. 201 West 4th Str., Suite 204, Claremont CA California, 91711, USA for free access to C-QSAR programme.
Notes
†Preliminary communication presented at 12th Panhellenic Pharmacochemistry Symposium, January 27–28, 2006, Patras, Greece, Book of Abstrs., p. 28.
References
- Murray DH, Mendez J, Brown SA. The natural coumarins: occurrence, chemistry and biochemistry. J. Wiley & Sons, N. York 1982
- O'Kennedy R, Thornes RD. Coumarins: Biology, applications and mode of action. J. Wiley & Sons, Chichester 1997
- Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activity. Curr Pharm Des 2004; 10: 3813–3833
- Dall'Acqua F, Vedaldi D. CRC handbook of organic photochemistry and photobiology, WM Horspool, PS Song. CRC Press, Boca Raton, FL 1995; 1341–1350
- Santana L, Uriarte E, Dalla Via L, Gia O. A new benzoangelicin with strong photobiological activity. Bioorg Med Chem Lett 2000; 10: 135–137
- Guiotto A, Chilin A, Manzini P, Dall'Acqua F, Bordin F, Rodighiero P. Synthesis and antiproliferative activity of furocoumarin isosters Il Farmaco. 1995; 50: 479–488
- Ploypradith P, Mahidol C, Sahakitpichan P, Wongbundit S, Ruchirawat S. A highly efficient synthesis of Lamellarins K and L by the Michael addition/ring-closure reaction of benzyldihydroisoquinoline derivatives with ethoxycarbonyl-β-nitrostyrenes. Angew Chem Int Ed 2004; 43: 866–868
- Meyer H. Studies on coumarins. Pyrrolocoumarins. Acta Chem Scand, Ser B 1975; 29: 133–140
- Khoshtariya TE, Kurkovsaya LN, Bochoidze LT, Batsikadze KT, Mirziashvili NT, Abesadze IG, Sikharulidze MI, Turabelidze VG, Ananiashvili VO, Dzhashi TO, Maisuradze MG. Pyrrolocoumarins. 1. Synthesis and spectroscopic investigation of 7-oxo- and 1,2,7-trioxo-1,2-dihydropyrano[3,2-e]indole and their derivatives. Chem Heterocyclic Comp 2005; 41: 208–215
- Suslov VV, Gordeev EN, Traven VF. Novel schemes for the synthesis of pyrrolocoumarins. Chem Heterocyclic Comp 2004; 40: 1315–1322
- Rodighiero P, Chili A, Pastorini G, Guiotto A. Pyrrolocoumarin derivatives as potential photoreagents toward DNA J Heterocyclic Chem 1987; 24: 1041–1043
- Khan MA, de B Morley ML. Condensed benzopyrans. IV. Synthesis of some derivatives of 7H- and 9H-pyrano[3,2-e]indoles. J Heterocyclic Chem 1979; 16: 997–999
- Majumdar KC, Ghosh SK. Studies on amine oxide rearrangements: Regioselective synthesis of pyrano[3,2-e]indol-7-one. J Chem Soc Perkin Trans 1994; 1: 2889–2894
- Gonzales JC, Lobo-Antunes J, Perez-Lourido P, Santana L, Uriarte E. Synthesis of angular pyrrolocoumarins Synthesis 2002; 475–478
- Hiremath SP, Badiger GR, Jivanagi AS, Purohit MG. Synthesis, spectral and biological studies of oxazinono and pyrono indoles. Ind J Chem 1992; B31: 583–589
- Hiremath SP, Badami PS, Purohit MG. Synthesis of substituted 1H-3,4-dihydro[1,2]diazepino[4,5,6-cd]indol-3-ones, 3,7-dihydropyrano[3,2-e]indol-7-ones and 6H-oxazolo[4,5-e]indoles. Indian J Chem 1983; B22: 437–440
- Nicolaides DN, Bezergiannidou-Balouctsi C, Wagih Awad R, Litinas KE, Malamidou-Xenikaki E, Terzis A, Raptopoulou CP. Site-selective Diels-Alder reactions of 7-(methoxyimino)-4-methylchromene-2,8-dione with alkenes. J Org Chem 1997; 62: 499–502
- Nicolaides DN, Fylaktakidou KC, Litinas KE, Adamopoulos SG. The synthesis of some pyrano[2,3-g]chromene-2,7-diones and furo[2,3-g]chromen-6-ones. J Heterocyclic Chem 1998; 35: 91–96
- Nicolaides DN, Gautam DR, Litinas KE, Manouras C, Fylaktakidou KC. Reactions of 2-(methoxyimino)benzen-1-ones with a-alkylethoxycarbonylmethylene(triphenyl)phosphoranes Tetrahedron 2001; 57: 9469–9474
- Nicolaides DN, Gautam DR, Litinas KE, Papamehael T. Synthesis of some 3,4-dihydro-2H-benzo[f]pyrano[2,3-h]chromen-6-one derivatives. J Chem Soc Perkin Trans 2002; 1: 1455–1460
- Fylaktakidou KC, Gautam DR, Hadjipavlou-Litina DJ, Kontogiorgis CA, Litinas KE, Nicolaides DN. Reactions of 4-methylchromene-2,7,8-trione with phosphonium ylides. Synthesis and evaluation of fused 1,3-dioxolocoumarins as antioxidants and antiinflammatories. J Chem Soc Perkin Trans 2001; 1: 3073–3079
- Kontogiorgis C, Hadjipavlou-Litina D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidants agents. J Enz Inhib Med Chem 2003; 18: 63–69
- Nicolaides DN, Gautam DR, Litinas KE, Hadjipavlou-Litina DJ, Fylaktakidou KC. Synthesis and evaluation of the antioxidant and anti-inflammatory activities of some benzo[l]khellactone derivatives and analogues. Eur J Med Chem 2004; 39: 323–332
- Kontogiorgis CA, Hadjipavlou-Litina DJ. Synthesis and anti-inflammatory activity of coumarin derivatives. J Med Chem 2005; 48: 6400–6408
- Candan F. Effect of Rhus coribum L. (Aracardiaceae) on superoxide radical scavenging and xanthine oxidase activity. J Enz Inhib Med Chem 2003; 18: 59–62
- Quick AJ. The prothrombin in hemophilia and in obstructive jaundice. J Biol Chem 1935; 109: 73–74
- Proctor RR, Rapaport SI. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Amer J Clin Path 1961; 36: 212–219
- Baldoumi V, Gautam DR, Litinas KE, Nicolaides DN. Convenient synthesis of linear pyrano[3,2-g]-, [2,3-g]- and angular pyrano[3,2-f]coumarins from 4[(1,1-dimethyl-2-propynyl)oxy]phenol Tetrahedron 2006; 62: 8016–8020
- Yavari I, Adib M, Hojabri L. Vinyltriphenylphosphonium salt mediated synthesis of 1,4-benzoxazine and coumarin derivatives. Tetrahedron 2002; 58: 6895–6899
- Yavari I, Anary-Abbasinejad M, Hossaini Z. Reaction between naphthols and dimethyl acetylene dicarboxylate in the presence of phosphites. Synthesis of stable oxa-2λ5-phosphaphenanthrenes, and benzochromene derivatives. Org Biomol Chem 2003; 1: 560–564
- Day RO, Graham GG, Williams KM, Brooks PM. Variability in response to NSAIDs. Fact or fiction? Drugs 1988; 643–651
- Ellis GA, Blake DR. Why are non-steroidal anti-inflammatory drugs so variable in their efficacy? A description of ion trapping. Ann Rheum Dis 1993; 241–243
- Muller K. 5-lipoxygennase and 12-lipoxygenase: attractive targets for the development of novel antipsoriatic drugs, reviews and trends. Archiv Pharm 1994; 327: 3–19
- Chapelev LL, Beshara CS, MacLean PD, Hartfield GL, Raud AA, Thompson A, Wright JS, Barclay LRC. Polypyrroles as antioxidants: Kinetic studies on reactions of bilirubin and biliverdin dimethyl esters and synthetic model compounds with peroxyl radicals in solution. Chemical calculations on selected typical structures. J Org Chem 2006; 71: 22–30
- Biobyte Corp., C-QSAR Database 201 West 4th Str., Suite 204, Claremont, CA, California 91711, USA.