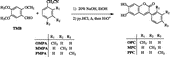Abstract
A new series of 6, 7-dihydroxy-3-(methylphenyl) chromenones, including three new derivatives, i.e. 6,7-dihydroxy-3-(2-methylphenyl)-2H-chromen-2-one (OPC); 6,7-dihydroxy-3-(3-methylphenyl)-2H-chromen-2-one (MPC); 6,7-dihydroxy-3-(4-methylphenyl)-2H-chromen-2-one (PPC) and one previously described, namely 6,7-dihydroxy-3-phenyl-2H-chromen-2-one (DPC), were synthesized. These compounds were investigated as inhibitors of human carbonic anhydrase I (hCA-I) and human carbonic anhydrase II (hCA-II) which had been purified from human erythrocytes on an affinity gel comprised of L-tyrosine-sulfonamide-Sepharose 4B.
Introduction
The involvement of the carbonic anhydrase (CA) enzyme family, which catalyzes the physiological hydration of CO2 to yield bicarbonate and a proton, in many physiological/pathological processes opens up widespread opportunities for the development of diverse, specific inhibitors for clinical application [Citation1,Citation2].
There is convincing evidence to suggest that the expression of CAs is increased in many tumours where their action in acidifying the extracellular milieu may give tumours a growth advantage over normal tissues [Citation3,Citation4]. Acetazolamide, a sulfonamide inhibitor of CA, has been shown to inhibit the in vitro invasion of renal cancer cells, which can provide additive delays in tumour growth when used in combination with other cytotoxic agents [Citation5,Citation6]. In view of the important role that CAs may have in supporting tumour growth there is currently considerable interest in developing CA inhibitors for use in cancer therapy [Citation7]. Inhibition of CA in the ciliary processes of the eye decreases aqueous humor secretion, presumably by slowing the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport, and is effective in the treatment of glaucoma [Citation8,Citation9]. Literature surveys reveal that sulfonamides and coumarin moieties are important pharmacophores and exhibit outstanding biological properties.
A wide spectrum of pharmacological activities are displayed by coumarins and their derivatives [Citation10,Citation11]. Coumarin derivatives are used widely as anticoagulants (such as warfarin, -OH group is attached at 4-position) for the treatment of disorders in which there is excessive or undesirable clotting, such as thrombophlebitis, pulmonary embolism, and certain cardiac conditions. They are also used as rodenticides due to their ability to cause fatal hemorrhaging [Citation12].
In biosynthesis the coumarin nucleus (benzo-2-pyrone) is derived from cinnamic acid (phenylacrylic skeleton). Hydroxylation at the 7-position is common in biosynthesis yielding such derivatives as umbelliferone (7-hydroxy coumarin), esculetin (6,7-Dihydroxycoumarin), and scopoletin (7-hydroxy-6-methoxycoumarin) which are the most widespread coumarins in nature. Synthetic 7-hydroxy coumarins are used to absorb ultraviolet rays in sunscreen cosmetics and are also used in the synthesis of drugs especially against cancer [Citation13].
In the present study we have synthesized derivatives of 6, 7-dihydroxy-3-(methylphenyl) chromenones for evaluation as potential inhibitors of cytosolic carbonic anhydrase isozymes that could be beneficial in the development of anti-glaucoma and anti-cancer therapies.
Materials and methods
All chemicals were from Sigma (USA) or Aldrich (Germany) and were used without further purification. 6, 7-Dihydroxy-3-phenyl-2H-chromen-2-one (DPC) was available from a previous study [Citation14].
The structures of all synthesized compounds were identified from their IR (Perkin Elmer Spectrum BX II) and 400 MHz 1H-NMR spectra (Bruker GmbH DPX-400). Melting points were measured on an Electrothermal 9200 instrument.
General procedure for the synthesis of 6, 7-dihydroxy-3-(methylphenyl) chromenones
Methyl-substituted coumarin compounds, i.e. the 6, 7-dihydroxy-3-(methylphenyl) chromenones, were synthesized by the procedure of Buu-Hoi et al. [Citation15]. A solution of 2, 3, 4-trimethoxybenzaldehyde (TMB) (25.0 mmol) and methylphenylacetonitrile (37.5 mmol) in ethanol (100 mL) was heated to 70°C. Aqueous NaOH (20%) was then added dropwise to the stirred solution until the onset of turbidity. The acrylonitrile which precipitated when the solution was cooled was washed with water by filtration. It was then treated with HCl and then H3O+. The precipitates were collected by filtration, and the dried product was purified by recrystallisation from ethanol (Scheme ).
6,7-dihydroxy-3-(2-methylphenyl)-2H-chromen-2-one (OPC)
2-Methylphenylacetonitrile (OMPA) was used as the starting compound. Yield, 76.5%; mp, 242°C; IR (KBr), ν (cm− 1): 3464(OH), 1655(C = O), 1297(C–H), 1151(C–O). 1H-NMR (CDCl3 + DMSO), δ (ppm): 2.20 (s, 3H, CH3), 6.83 (s, H, ArH), 6.98 (s, H, ArH), 7.32 (m, 4H, ArH), 7.50 (s, H, cumH), 7.86 (s, 2H, OH).
6,7-dihydroxy-3-(3-methylphenyl)-2H-chromen-2-one (MPC)
3-Methylphenylacetonitrile (MMPA) was used as the starting compound. Yield, 76.8%; mp, 211°C; IR (KBr), ν (cm− 1): 3172(OH), 1669(C = O), 1258(C–H), 1168(C–O). 1H-NMR (CDCl3 + DMSO), δ (ppm): 2.32 (s, 3H, CH3), 6.75 (s, H, ArH), 6.91 (s, H, ArH), 7.08 (d, H, ArH), 7.21 (d, H, ArH), 7.37 (d, 2H, ArH), 7.70 (s, H, cumH), 7.75 (s, 2H, OH).
6,7-dihydroxy-3-(4-methylphenyl)-2H-chromen-2-one (PPC)
4-Methylphenylacetonitrile (PMPA) was used as the starting compound. Yield, 80.5%; mp, 257-258°C; IR (KBr), ν (cm− 1): 3151(OH), 1660(C = O), 1273(C–H), 1188(C–O). 1H-NMR (CDCl3 + DMSO), δ (ppm): 2.30 (s, 3H, CH3), 6.75 (s, H, ArH), 6.90 (s, H, ArH), 7.13 (d, 2H, ArH), 7.48 (d, 2H, ArH), 7.67 (s, H, cumH), 7.73 (s, 2H, OH).
Preparation of hemolysates
Blood samples (25 mL) were taken from healthy human volunteers. They were anticoagulated with ACD (acid-citrate-dextrose), centrifuged at 1848 × g for 20 min at 4°C and the supernatant was removed. The packed erythrocytes were washed three times with 0.9% NaCI and then hemolysed in cold water. The ghosts and any intact cells were removed by centrifugation at 18924 × g for 25 min at 4°C, and the pH of the hemolysate was adjusted to pH 8.5 with solid Tris-base. The 25 mL hemolysate was applied to an affinity column containing L-tyrosine-sulfonamide-Sepharose-4B [Citation16,Citation17] equilibrated with 25 mM Tris–HCl / 0.1 M Na2SO4 (pH 8.5). The affinity gel was washed with 50 mL of 25 mM Tris–HCl / 22 mM Na2SO4 (pH 8.5). The human CA (hCA) isozymes were then eluted with 0.1 M NaCl / 25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa / 0.5 M NaClO4 (pH 5.6), which recovered hCA-I and hCA-II respectively. Fractions of 3 mL were collected and their absorbance measured at 280 nm.
Determination of protein content
Quantitative protein determination was performed on the pooled peaks of the eluted hCAs using the Coomassie Brilliant Blue G-250 method [Citation18].
Enzyme assay and inhibition studies
CA enzyme activity was assayed both with respect to its hydratase activity (i.e. hydration of CO2 [Citation19]), and to its esterase activity (i.e. hydrolysis of an ester of p-nitrophenylacetate [Citation20]).
The Maren method [Citation19] is based on determination of the time required for the pH of a standard solution to decrease from pH 10.0 to 7.4 due to CO2 hydration. The assay solution was 0.5 M Na2CO3/0.1M NaHCO3 (pH 10.0) and Phenol Red was added as the pH indicator. One unit of CA activity is defined as that amount of the enzyme that reduces by 50% the time for CO2 hydration measured in the absence of enzyme. The second hydrolytic method measures CA activity using p-nitrophenylacetate as a substrate followed by monitoring at 348 nm [Citation20].
The inhibition of CA activity by the various coumarin derivatives (DPC, OPC, OPC and PPC) was studied by determining their effects on the CA-catalyzed CO2 hydration rate at 1°C. CO2 hydration rates were determined at five different inhibitor concentrations using an initial substrate concentration of 70 mM. Regression analysis was carried out on the graph of percent inhibition values as a function of inhibitor concentration.
Results and discussion
Carbonic anhydrase (CA) is a common enzyme that is present in key regulatory organs. By catalyzing the interconversion between carbon dioxide and bicarbonate, with generation of a proton, CAs operate on three very simple molecules/ions involved in a variety of critical life processes [Citation1,Citation2] Among them, the most important ones are pH regulation, respiration, secretion of electrolytes, biosynthesis of some important biomolecules such as urea, glucose, lipids, and pyrimidines, excretion of acid and salts, carcinogenesis, and cell signaling [Citation20,Citation21]. In the kidney, CA is involved in regulating acid-base equilibrium [Citation22]. Its action in the renal tubules produces a mild metabolic acidosis by increasing bicarbonate excretion, which increases pulmonary ventilation. In the lungs, erythrocyte CA is responsible for the dehydration of H2CO3 and its hydration in the tissues. Without a catalyst, the interconversion between CO2 and H2CO3 requires more than 1 minute for completion, while the capillary transit of erythrocytes takes 1 second. Inhibition of CA by acetazolamide is used as a prophylactic treatment for acute mountain sickness [Citation23]. It is known that this substance produces an elevation of the arterial PO2 during acute exposure to hypoxia. It is also an effective inhibitor in the treatment of glaucoma. Therapeutic inhibition of CA is thus feasible and effective, and new inhibitors with different properties and/or potencies are potentially valuable.
Coumarins are active components of herbs used for the treatment of various diseases [Citation24]. In the literature, two coumarin-based sulfamate drugs (667 COUMATE and STX 118) have been reported to have IC50 values of 25–59 nM for the inhibition of hCA-II activity. These compounds therefore have similar CA-II inhibitory potencies to that of acetazolamide (IC50 25 nM), the well-known hCA-II inhibitor [Citation25]. In the literature, many sulfonamide derivatives have been described which inhibit members of the CA family Citation27-30. Synthesis of coumarin 3-(N-aryl) sulfonamides has been accomplished either by Knoevenagel condensation of anilinosulfonylacetic acids with suitable salicylaldehydes, or by the reaction of methyl anilinosulfonylacetates with substituted salicylaldehydes in the presence of a catalytic amount of a base. All the compounds tested for antiproliferative activity in different cancer cell lines have shown GI50 values less than 100 μM [Citation31]. Supuran and his group have reported that some of the coumarinyl-substituted benzenesulfonamide series are potent, low nanomolar CA-V inhibitors [Citation26]. In another study, Zobel et al. demonstrated the enhanced anti-carcinogenic potential of combining coumarin at high concentrations (greater than 10 ppm) with the chemotherapy agent, Paclitaxel [Citation32].
Recently, new synthetic routes to this class of compounds have been reported, also detailing their variable and interesting biological and pharmacological activities Citation10-13. There have also been very interesting studies of the synthesis and selective biological properties of 7-hydroxy-4-methyl-2H-chromen-2-one (2), 7-hydroxy-4,5-dimethyl-2H-chromen-2-one (15) and some of their derivatives [Citation10]. These stimulated us to synthesize a number of new dihydroxy coumarin compounds for testing against hCA-I and -II isozymes.
These new coumarin compounds were tested for their ability to modulate both the hydratase and esterase activities of cytosolic CA isozymes. Their in vitro inhibitory effects on hCA-I and hCA-II, purified by affinity chromatography from erythrocytes, are summarised in . The hCA-II isozyme evolved to very efficiently catalyze the reversible hydration of CO2, but it also exhibits promiscuous activity toward highly activated esters such as 4-nitrophenyl acetate. Among the compounds tested, MPC was the least effective inhibitor of hCA-I, exhibiting IC50 values of 0.837 mM and 0.320 mM against esterase and hydratase activities, respectively. For hCA-II, DPC was the weakest inhibitor of esterase activity (IC50 of 0.246 mM), but PPC was the weakest hydratase inhibitor (IC50 of 0.561 mM), closely followed by MPC (IC50 of 0.504 mM). The most effective inhibitor of hCA-I esterase activity was OPC (IC50 of 0.153 mM) and that of hCA-II esterase activity was PPC (IC50 of 0.127 mM). In contrast, the most potent inhibitor of hydratase activities was DPC with both hCA-I and –II isozymes (IC50 values of 0.124 mM and 0.0617 mM, respectively). Lindskog et al. [Citation33] had previously found that the phenol is a weaker inhibitor for CA I and CA II and KI values for CA-II with hydratase activity as 18 mM and for esterase activity was 30 mM.In the present study with the new compounds belonging to the diphenol class, we observed a better inhibition of the two CA isozymes with them, as compared to the simple phenol investigated earlier ().
Table I. IC50 values of cytosolic CA isozymes I and II with the phenold dscribed in the paper.
Our group had also previously synthesized an original sulfonamide derivative of coumarin, namely 2-(8-methoxycoumarine-3-carbamido)-1,3,4-thiadiazole-5-sulfonamide (MCTS). This compound showed IC50 values of 0.0047, 0.0096 and 0.0253 mM against the hydratase activities of hCA-I, hCA-II and dog carbonic anhydrase (dCA), respectively [Citation9,Citation26].
Inhibitors of CA play an important role in ophthalmology, where they are used to reduce elevated intraocular pressures. Apart from transient myopia and blurred vision, no adverse reactions from the eye have been described in spite of long-term treatment of patients over many years. The dihydroxy-coumarin derivatives described here do not exhibit the inhibitory potencies of acetazolamide, or even that of the coumarin-sulfonamide MCTS. However, they do demonstrate that this synthetic route has some potential that is worth further investigation in the search for more therapeutically-effective CA inhibitors for potential use in the treatment of glaucoma, or other pathologies, where acidification by carbonic anhydrases plays a role.
Acknowledgements
The authors would like to thank Balikesir University Research Center of Applied Sciences (BURCAS/Balikesir,Turkey) for providing the research facilities and also thank to Dr. Malcolm LYON for his invaluable contribution to this paper.
References
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors. Curr Med Chem-Imm Endoc Metab Agents 2001; 1: 61–97
- Thiry A, Dogné JM, Masereel B, Supuran CT. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 2006; 27: 566–573
- McKierman JM, Buttyan R, Stifelman MD, Kutz AG, Chen MW, Olsson CA, Sawczuk IS. Expression of tumor-associated gene MN: A potential biomarker of human renal cell carcinoma. Cancer Res 1997; 57: 2362–2365
- Tureci O, Sahin O, Vollmar E, Siemer S, Gottert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS. Human carbonic anhydrase XII: cDNA cloning, expression and chromosomal localization of a carbonic anhydrase gene that is over expressed in some renal cancers. Proc Natl Acad Sci USA 1998; 95: 7608–7613
- Parkkila S, Rajaniemi H, Parkkila AK, Kivelä J, Waheed A, Pastorekova S, PAstorek J, Sly WS. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci 2000; 97: 2220–2224, USA
- Teicher BA, Liu SD, Liu JT, Holden SA, Herman TS. A carbonic anhydrase inhibitor as a potential modulator of cancer therapies. Anti Cancer Res 1993; 13: 1549–1556
- Ozensoy O, Puccetti L, Fasolis G, Arslan O, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of the tumor associated isozymes IX and XII with a library of aromatic and heteroaromatic sulfonamides. Bioorgan Med Chem Lett 2005; 15(21)4862–4866
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003; 23: 146–189
- Bayram T, Arslan O, Ugras HI, Cakır U, Ozensoy O. Purification of carbonic anhydrase from dog erytrocytes and investigation of in vitro inhibition effects by various compounds. J Enz Inhib Med Chem 2007, in press
- Khan KM, Szaify ZS, Khan M, Ullah Z, Choudhary IM, Rahman AU, Perveen S, Chohan ZH, Supuran CT. Synthesis of coumarin derivatives with cytotoxic, antibacterial and antifungal activity. J Enz Inhib Med Chem 2004; 19(4)373–379
- Rehman SU, Chohan ZH, Gulnaz F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of somecoumarins and their metal complexes. J Enz Inhib Med Chem 2005; 20(4)333–340
- Budzisz E. Synthesis, reactions and biological activity of phosphorus-containing derivatives of chromone and coumarin. Phosphorus, sulfur and silicon and the related elements. 2004; 179(10)2131–2147(17)
- Iscan M, Rostami H, Güray T, Pelkonen O, Rautio A. Interindividual variability of coumarin 7-hydroxylation in a turkish population. Eur J Clin Pharmacol 1994; 47(4)315–318
- Cakir U, Ozer M, Icen MA, Ugras HI, Bulut M. Synthesis of some 3-phenyl chromenone-crown ethers and equilibrium studies on complexation with ion-pair extraction of sodium and potassium dyes. Dyes Pigments 2004; 60: 177–185
- Buu-Hoi NP, Saint-Ruf G, Lobert B. Oxygen heterocycles. Part XIV. Hydroxylated 3-aryl-and 3-pyridyl-coumarins. J Chem Soc C 1969; 16: 2069
- Ozensoy O, Arslan O, Oznur Sinan S. A new method for purification of carbonic anhydrase isozymes by affinity chromatography. Biochemistry (Moscow) 2004; 69: 216–219
- Isık S, Kockar F, Ozensoy O, Arslan O. Carbonic anhydrase from cyprinus carpio fresenius. Envir Bull 2004; 13(1)25–29
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Expert Opin Ther Pat 2006; 16: 1627–1644
- Mc Intosh J. Carbonic anhydrase isoenzymes in the erythrocytes and dorsolateral prostate of the rat. J Biochem 1969; 14: 463–467
- Gamboa J, Caceda R, Gamboa A, Monge-C C. Carbonic anhydrase activity in the red blood cells of sea level and high altitude natives. Biol Res 2000; 33(3–4)207–208
- Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: Sulfonamides as antitumor agents?. Bioorg Med Chem 2001; 9(3)703–714
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin Ther Pat 2000; 10(5)575–600
- Okamoto T, Kobayashi T, Yoshida S. Chemical aspects of coumarin compounds for the prevention of hepatocellular carcinomas current medicinal chemistry - anti-cancer agents. 2005; 5: 47–51
- Leese MP, Leblond B, Smith A, Newman SP, Di Fiore A, De Simone G, Supuran CT, Purohit A, Reed MJ, Potter BV. 2-Substituted estradiol bis-sulfamates, multitargeted antitumor agents: Synthesis, in vitro SAR, protein crystallography, and in vivo activity. J Med Chem 2006; 49: 7683–7696
- Vullo D, Franchi M, Gallori E, Antel J, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mitochondrial Isozyme V with Aromatic and heterocyclic sulfonamides. J Med Chem 2004; 47: 1272–1279
- Cakir U, Ugras HI, Ozensoy O, Sinan S, Arslan O. In vitro effects of some new sulfonamide Inhibitors on human carbonic anhydrase I and II. J Enz Inhib Med Chem 2004; 19(3)257–261
- Menchise V, Simone De G, Fiore A Di, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: X-ray crystallographic studies for the binding of 5-amino-1,3,4-thiadiazole-2-sulfonamide and 5-(4-amino-3-chloro-5-fluorophenylsulfonamido)-1,3,4-thiadiazole-2- sulfonamide to human isoform II. Bioorg Med Chem Lett 2006; 16(24)6204–6208
- Nishimori I, Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition studies of the human secretory isoform VI with anions. Bioorg Med Chem Lett 2007; 17(4)1037–1042
- Temperini C, Innocenti A, Scozzafava A, Supuran CT. N-Hydroxyurea—A versatile zinc binding function in the design of metalloenzyme inhibitors. Bioorgan Med Chem Lett 2006; 16: 4316–4320
- Srinivasa RN, Mallireddigari MR, Cosenza S, Gumireddy K, Bell SC, Prekumar RE, Ramana RMV. Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity. Bioorg Med Chem Lett 2004; 14(15)4093–4097
- Zobel AM, Schellenberger SE. Paclitaxel in combination with coumarin as a potentially effective anticancer agent. Pharm Biol 2000; 38(3)192–196
- Simonsson I, Jonsson BH, Lindskog S. Phenol, a competitive inhibitor of CO2 Hydration catalyzed by carbonic anhydrase. Biochem Biophys Res 1982; 108(4)1406–1412