Abstract
A series of efavirenz Mannich bases has been synthesized by reacting efavirenz, formaldehyde, and various aryl substituted piperazines using microwave irradiation (yield 35–88%). The synthesized compounds were evaluated for in-vitro anti-HIV and antimycobacterial activities. The in-vitro antiretroviral activities indicated that compound 7-(4-((6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2-oxo-2H-benzo[d] [1,3]oxazin-1 (4H)-yl)methyl)-3-methylpiperazin-l -yl)-1-cyclopropyl-6-fluoro-l,4-dihydro-8-methoxy-4-oxoquinoline-3-carboxylic acid (4i) was equipotent to efavirenz with EC50 of 2.4 nM. Compound 4i also inhibited M. tuberculosis with minimum inhibitory concentration of 0.2 μg/mL.
Indroduction
Acquired immunodeficiency syndrome (AIDS) is caused by the retrovirus, human immuno deficiency virus (HIV) [Citation1]. The number of people living with HIV continues to grow and around 40 million peoples are infected with HIV globally, and over 20 million have died since the first cases of AIDS were identified in 1981 [Citation2]. The HIV infection, which targets the monocytes expressing surface CD4 receptors, eventually produces profound defects in cell-mediated immunity [Citation3]. Overtime infection leads to severe depletion of CD4 T-lymphocytes (T-cells) resulting in opportunistic infections (OI) like tuberculosis (TB), fungal, viral, protozoal and neoplastic diseases and ultimately death. Worldwide, TB is the most frequent co-infection in subjects with HIV type 1 infection [Citation4]. HIV-1 infection remains the most common risk factor for the development of active TB [Citation5]. Both reactivation of a latent Mycobacterium tuberculosis (MTB) infection and progressive primary TB are substantially more common in HIV-1-infected subjects [Citation6]. The development of active TB accelerates the progression of HIV disease towards full-blown AIDS, accompanied by enhanced HIV replication. Through rational thinking, it appears that an ideal drug for HIV/AIDS patients should suppress HIV replication thereby acting as anti-HIV drug and also should treat OI like TB [Citation7–8]. In continuation of our work on anti-HIV prodrugs [Citation9–13], we undertook a study to prepare and evaluate efavirenz prodrugs in an effort to identify compounds which could suppress HIV-replication and also inhibit MTB.
Materials and Methods
Chemistry
A domestic microwave oven with the following specifications has been used: make LG; input 220 V ∼ 50 Hz, 980 W, 4.7°A; frequency 2450 MHz. Melting points were determined in open capillary tubes on a Büchi 530 melting point apparatus and are uncorrected. Infrared (IR) and proton nuclear magnetic resonance (1H-NMR) spectra were recorded for the compounds on a Jasco IR Report 100 (KBr) and Brucker Avance (300 MHz) instruments, respectively. Chemical shifts are reported in parts per million (ppm) using tetramethyl silane (TMS) as an internal standard. Elemental analyses (C, H, and N) were undertaken with a Perkin-Elmer model 240C analyzer. The homogeneity of the compounds was monitored by ascending thin layer chromatography (TLC) on silicagel-G (Merck) coated aluminium plates, visualized by iodine vapour. Developing solvents were chloroform-methanol (9:1).
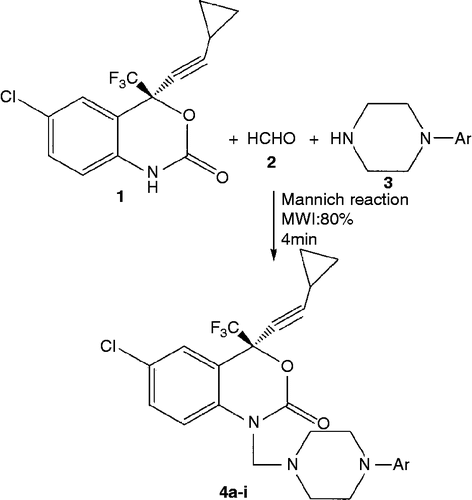
Synthesis of Compounds 4a-i
The general procedure for preparing 4a-i was as follows. To a solution of (6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-lH-benzo[d][l,3]oxazin-2-(4H)-one) (efavirenz, 0.02 mol) in absolute ethanol (50 mL), was added various aryl piperazine derivatives (0.02 mol) and 37% formalin (1 mL). The reaction mixture was irradiated in an unmodified domestic microwave oven at 80% intensity with 30 s/cycle for 3 min and set aside. The resultant solid was washed with dilute ethanol dried and recrystallized from ethanol-chloroform mixture. Yield 35–88%.
Spectral and elemental analysis data for representative compounds
6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-l-((4-phenylpiperazin-l-yl)methyl)-lH-benzo[d][l,3]oxazin-2(4H)-one (4a):
IR (KBr): 3010, 2862, 2840, 1742, 1640, 1620, 1506, 1240, 1125 cm− 1; 1H-NMR (DMSO-d6) δ (ppm): 0.7–0.82 (m, 4H, cyclopropyl-H of efavirenz), 1.58 (m, IH, cyclopropyl-H of efavirenz), 2.6–3.45 (m, 8H, -piperazine-H), 4.0 (s, 2H, -NCH2N), 5.1 (m, IH, CH of ethylene linker attached to cyclopropyl), 6.0 (d, IH, CH of ethylene linker attached to benzoxazine), 6.59–7.54 (m, 8H, Ar-H); Calculated for C25H25C1F3N302: C, 61.04; H, 5.12; N, 8.54. Found: C, 61.01; H,4.15;N, 8.59%.
6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-l-((4-(4-methoxyphenyl)piperazin-1 -yl)methyl)-l H-benzo[d][1,3]oxazin-2(4H)-one (4c):
IR (KBr): 3010, 2860, 2842, 1742, 1640, 1620, 1510, 1240, 1125 cm− 1; 1H-NMR (DMSO-d6) δ (ppm): 0.7–0.80 (m, 4H, cyclopropyl-H of efavirenz), 1.58 (m, IH, cyclopropyl-H of efavirenz), 2.62–3.46 (m, 8H, -piperazine-H), 3.73 (s, 3H, methoxy), 4.02 (s, 2H, -NCH2N), 5.1 (m, IH, CH of ethylene linker attached to cyclopropyl), 6.02 (d, IH, CH of ethylene linker attached to benzoxazine), 6.6–7.54 (m, 7H, Ar-H); Calculated for C26H27CIF3N3O3: C, 59.83; H, 5.21; N, 8.05. Found: C, 59.90; H, 5.19; N, 8.09%.
7-(4-((6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-2-oxo-2H-benzo[d] [1,3]oxazin-1 (4H)-yl)methyl)-3 -methylpiperazin-1 -yl)-6,8-difluoro-1 -ethyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acid (4f):
IR (KBr): 3010, 2860, 2846, 1740, 1640, 1620, 1506, 1236, 1125 cm− 1; 1H-NMR (DMSO-d6) δ (ppm): 0.7–0.82 (m, 4H, cyclopropyl-H of efavirenz), 1.28 (t, 3H, CH3 of C2H5), 1.58 (m, IH, cyclopropyl-H of efavirenz), 2.7–3.6 (m, 8H, -piperazine-H), 4.03 (s, 2H, -NCH2N), 4.25 (q, 2H, CH2 of C2H5), 5.1 (m, IH, CH of ethylene linker attached to cyclopropyl), 6.0 (d, IH, CH of ethylene linker attached to benzoxazine), 6.60–7.51 (m, 5H, Ar-H), 8.0 (s, IH, C2-H), 14.60 (bs, IH, COOH); Calculated for C31H29CIF4N4O5: C, 57.37; H, 4.50; N, 8.63. Found: C, 57.39; H, 4.49; N, 8.60%.
7-(4-((6-chloro-4-(2-cycIopropylethynyl)-4-(trifluoromethyl)-2-oxo-2H-benzo[d][l,3]oxazin-l(4H)-yl)methyl)-3-methylpiperazin-l-yl)-l-cyclopropyl-6-fluoro-l,4-dihydro-8-methoxy-4-oxoquinoline-3-carboxylic acid (4i):
IR (KBr): 3010, 2860, 2846, 1740, 1640, 1620, 1506, 1236, 1125 cm− 1; 1H-NMR (DMSO-d6) δ (ppm): 0.28–0.53 (m, 4H, cyclopropyl-H of quinolone), 0.7–0.82 (m, 4H, cyclopropyl-H of efavirenz), 1.2 (s, 3H, CH3 of piperazine), 1.4 (m, IH, cyclopropyl-H of quinolone), 1.58 m, IH, cyclopropyl-H of efavirenz), 2.6–3.4 (m, 7H,-piperazine-H), 3.73 (s, 3H, methoxy), 4.03 (s, 2H, -NCH2N), 5.1 (m, IH, CH of ethylene linker attached to cyclopropyl), 6.0 (d, IH, CH of ethylene linker attached to benzoxazine), 6.60–7.7 (m, 4H, Ar-H), 8.0 (s, IH, C2-H), 14.86 (bs, IH, COOH); Calculated for C34H33CIF4N4O6:C, 57.92; H, 4.72; N, 7.95. Found: C, 57.91; H, 4.69; N, 7.90%.
Anti-HIV activity
The compounds were tested for anti-HIV activity against replication of HIV-1 (III B) in CEM cells [Citation14]. The CEM cells were grown in RPMI-1640 DM (Dutch modification) medium (Flow lab, Irvine Scotland), supplemented with 10% (v/v) heat-inactivated calf serum and 20-μg/mL gentamicin (E. Merck, Darmstadt, Germany). HIV-1 (III B) was obtained from the culture supernatant of HIV-1 infected MT-4 cell lines and the virus stocks were stored at − 70° C until used. Anti- HIV assays were carried out in microtitre plates filled with 100 μL of medium and 25 μL volumes of compounds in triplicate so asto allow simultaneous evaluation of their effects on HIV and mock infected cells. Fifty microlitres of HIV at 100 CCID50 (50% cell culture infective dose) medium were added to either the HIV infected or mock infected part of the microtitre tray. The cell cultures were incubated at 37 °C in a humidified atmosphere of 5% CO2 in air. Five days after infection the viability of mock and HIV-infected cells were examined spectrophotometrically by using the dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method [Citation8].
In vitro antimycobacterial activity (Agar dilution method) [Citation15]
Compounds were evaluated for their in vitro antimycobacterial activity against M. tuberculosis H37Rv procured from the Tuberculosis Research Center, Indian Council of Medical Research, Chennai, India. The agar dilution method was performed using Middlebrook 7H10 medium supplemented with Middlebrook OADC medium (Hi-Media). After solidification of the agar, the plates were inoculated with 0.1 mL of 10− 2 and 10− 4 dilutions of a McFarland 1.0 concentration of a suspension of organism. The inoculated plates were then incubated at 37° C for 4 weeks. The minimum inhibitory concentration (MIC) was considered to be the lowest concentration that completely inhibited growth on agar plates, disregarding a single colony or a faint haze caused by the inoculums.
In vitro stability studies [Citation16]
To 990 μL of phosphate buffer (0.2 M, pH 7.4) was added 10 μL of a solution of appropriate drugs (10 mg/mL in dimethyl sulfoxide) and the mixture was incubated at 37 °C in a water bath. At various time intervals (0–4 h), 100 μL of the samples were withdrawn and added immediately to ice-cold methanol (400 μL). The samples were centrifuged and the supernatants were filtered through nylon 66 filters (0.45 pm) and analyzed by spectrophotometrically. From the observed absorbance changes, at various wavelengths, the half-lives of the analogues were calculated.
Results and Discussion
Synthesis and Characterisation
Efavirenz (6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-lH-benzo[d] [1,3] oxazin-2-(4H)-one), is a very potent HIV-1 non-nucleoside reverse transcriptase inhibitor [Citation17]. T Toll date, efavirenz has been modified at the 4-, 5- and 6-positions of the benzoxazine moiety [Citation18], and in this work we modified the amide hydrogen at the 1-position. The conversion of compounds containing an active hydrogen atom to the corresponding Mannich bases has been used extensively in the molecular modification approach [Citation19]. Here we prepared the Mannich bases of efavirenz by reacting it with formaldehyde and secondary amine function of substituted piperazines utilizing microwave irradiation to form the required efavirenz derivatives in 35–88% yield. The general proceduce for the preparation of target compounds 4a-4i () is described in . Unlike conventional methods (duration-5 h) [Citation17], microwave-assisted reactions were very facile (3-4 min), and the products do not require any further purification. The purity of the synthesized compounds was checked by thin layer chromatography (TLC) and elemental analyses and the structures were confuted by spectral data. In general, infra red spectra (IR) revealed a CH2 (Mannich methylene) peak at 2860 and 2846 cm− 1. In the lH NMR spectra the signals of the respective protons of the prepared efavirenz derivatives were verified on the basis of their chemical shifts, multiplicities and coupling constants. The spectra showed a singlet at δ 4.0–4.03 ppm corresponding to the-NCH2N- group; a multiplet at 0.7–0.82 and 1.58 ppm for the cyclopropyl proton; a multiplet at δ 2.59–3.45 ppm for the piperazine proton; a multiplet at δ 6.99–7.4 ppm for the aromatic protons. The absence of a D2O exchangeable singlet at 11.05 ppm indicated that the active hydrogen of efavirenz was substituted with the aminomethyl group. The elemental analysis results were within ± 0.4% of the theoretical values.
Table I. Physical constants and biological activities of efavirenz Mannich bases.
Biological investigation and discussion
The synthesized compounds were evaluated for their inhibitory effect on the replication of HIV-1 in CEM cell lines and their EC50 (effective concentration of compound (nM) achieving 50% protection in CEM lines against the cytopathic effect of HIV-1), and CC50 (cytotoxic concentration of compound (μM) required to reduce the viability of mock infected CEM cells by 50%), are reported in the along with efavirenz as standard drug for comparison. Examination of the obtained results revealed that compounds 4a-4i exhibited excellent anti-HIV activity. Among the synthesized compounds, three compounds (4e, 4f, and 4i) were found to be equally active as efavirenz. Compound 4i was found to be the most potent compound with EC50 of 2.4 nM and CC50 of >10 μM with selectivity index (CC50/EC50) of >4166.
All the compounds were screened for their antimycobacterial activity against MTB by the agar dilution method similar to that recommended by the National Committee for Clinical Laboratory Standards [Citation15] for the determination of minimum inhibitory concentration (MIC). The MIC was defined as the minimum concentration of compound required to inhibit complete growth of bacteria and MIC's of the compounds were reported in . Among the derivatives tested, compounds bearing the fluroquinolone moiety were found to be promising and compound 4i was found to the most active compound with MIC of 0.2 μg/mL. This enhanced activity might be due to the inhibition of MTB DNA gyrase by the compounds 4f-i [Citation20].
The usefulness of the prodrugs of efavirenz should depend not only on the stability of the prodrug for its transport across the cell membrane but also upon its reversion to the parent compound intracellularly, especially in the virally infected cells. The half-lives (t1/2) of hydrolysis of the prodrugs (4e-f, 4i) were therefore determined at pH 7.4, 37°C. The data in indicated that the various prodrugs of efavirenz were susceptible to hydrolysis with t1/2 in the range of 120–240 min through deaminomethylation to release efavirenz.
This study revealed that compound 4i was found to be a promising compound for the treatment of AIDS, as shown by excellent anti-HIV and antimycobacterial activity. Compound 4i suppresses HIV replication thereby acting as an anti-HIV drug and also could be useful to treat OI like TB.
Acknowledgements
The authors are thankful to the Indian Council of Medical Research [No. 58/9/2003-BMS] for their financial assistance and Dr. Vanaja Kumar, Deputy Director, Tuberculosis Research Center, Chennai, India for their assistance in biological screening.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- S Broder, and RC Gallo. (1984). A pathogenic retrovirus (HTLV-III) linked to AIDS. N Engl J Med 311:1292–1297.
- http:www.unaids.org.
- DL Bowen, HC Hane, and AC Fauci. (1985). Immunopathogenesis of the acquired immunodeficiency syndrome. Ann Intern Med 163:704–709.
- C Dye, S Scheele, P Dolin, V Pathania, and MC Raviglione. (1999). Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677–686.
- PA Selwyn, D Hartel, W Wasserman, and E Drucker. (1989). Impact of the AIDS epidemic on morbidity and mortality among intravenous drug users in a New York City methadone maintenance program. Am J Public Health 79:1358–1362.
- CL Daley, PM Small, and GF Schecter. (1992). An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med 326:231–235.
- D Sriram, and P Yogeeswari. (2003). Towards the design and development of agents with broad spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Curr Med Chem 10:1689–1695.
- D Sriram, TR Bal, and P Yogeeswari. (2004). Design, synthesis and biological evaluation of novel non-nucleoside HIV-1 reverse transcriptase inhibitors with broad-spectrum chemotherapeutic properties. Bioorg Chem 12:5865–5873.
- D Sriram, P Yogeeeswari, N Srichakravarthy, and TR Bal. (2004). Synthesis of stavudine amino acid ester prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Bioorg Med Chem Lett 14:1085–1087.
- D Sriram, P Yogeeswari, and G Gopal. (2005). Synthesis, anti-HIV and antitubercular activities of lamivudine prodrugs. Eur J Med Chem 40:1373–1376.
- D Sriram, N Srichakravarthy, TR Bal, and P Yogeeswari. (2005). Synthesis of zidovudine prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Biomed Pharmacother 59:452–455.
- D Sriram, N Srichakravarthy, TR Bal, and P Yogeeswari. (2005). Nevirapine derivatives with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Biomed Pharmacother 59:456–459.
- D Sriram, P Yogeeswari, NS Myneedu, and V Saraswat. (2006). Abacavir prodrugs: microwave-assisted synthesis and their evaluation of anti-HIV activities. Bioorg Med Chem 16:2127–2129.
- D Sriram, TR Bal, and P Yogeeswari. (2005). Aminopyrimidinimino isatin analogues: design of novel non- nucleoside HIV-1 reverse transcriptase inhibitors with broad-spectrum chemotherapeutic properties. Med Chem 20:565–577.
- National Committee for Clinical Laboratory Standards. Antimycobacterial susceptibility testing. Proposed standard M24-P. Villanova, Pa: National Committee for Clinical Laboratory Standards; (1990).
- SK Agarwal, SR Gogu, SRS Rangan, and KC Agrawal. (1990). Synthesis and biological evaluation of prodrugs of zidovudine. J Med Chem 33:1505–1509.
- SD Young, SF Britcher, LO Tran, LS Payne, WC Lumma, TA Lyle, JR Huff, PS Anderson, DB Olsenn, SS Carroll, DJ Pettibone, JA O'Brien, RG Ball, SK Balani, JH Lin, IW Chen, WA Schleif, VV Sardana, WJ Long, VW Brynes, and EA Emini. (1995). L-743, 726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother 39:2602–2605.
- M Patel, SS Ko, RJ McHugh, JA Markwalder, AS Srivastava, BC Cordova, RM Klabe, S Erickson-Viitanen, GL Trainor, and SP Seit. (1999). Synthesis and evaluation of analogs of Efavirenz (SUSTIVA) as HIV-1 reverse transcriptase inhibitors. Bioorg Med Chem Lett 9:2805–2810.
- P Yogeeswari, D Sriram, R Kavya, and S Tiwari. (2005). Synthesis and in-vitro cytotoxicity evaluation of gatifloxacin Mannich bases. Biomed Pharmacother 59:501–510.
- D Sriram, P Yogeeswari, SJ Basha, D Radha, and V Nagaraja. (2005). Synthesis and antimycobacterial evaluation of various 7-substituted ciprofloxacin derivatives. Bioorg Med Chem 13:5774–5778.