Abstract
The ethanol is a widely consumed as sedative-hypnotic drug throughout the world. In this study, the effects of ethanol were investigated on carbonic anhydrase (CA) enzyme activities both in vitro in human erythrocyte and in vivo in Sprague-Dawley rat erythrocyte. For in vitro study, the human carbonic anhydrase-I (HCA-I) and -II (HCA-II) are purified by Sepharose 4B–L-tyrosine-sulphanilamide affinity chromatography. In vivo CA enzyme activity was determined colorimetrically by using CO2-hydration method of Wilbur and Anderson. Rat blood samples were taken from each rat before and after the ethanol administration at different times (1 h, 3 h, and 5 h). Rat erythrocyte CA activity was significantly inhibited by pharmacological dosage of the ethanol (2 mL.kg− 1) for up to 3 h (p < 0.001) following intraperitoneally administration. The ethanol showed in vitro inhibitory effects on HCA-I and HCA-II hydratase activity, determined by colorimetrically using the CO2-hydratase method. The inhibitor concentrations causing up to 50% inhibition (IC50) were 2.09 M for HCA-I (r2:0.9273) and 1.83 M for HCA-II (r2:9749). In conclusion, it was demonstrated that carbonic anhydrase enzyme in erythrocytes was significantly inhibited by the ethanol both in in vitro and in vivo.
Keywords::
Introduction
Carbonic anhydrase (CA; Carbonate hydrolyase, EC 4.2.1.1) zinc containing metalloenzymes is a ubiquitous enzyme present in organisms belonging to all branches of the evolutionary tree, from archaea to mammals [Citation1] and is a well-characterized pH regulatory enzyme in most tissues. It catalyzes the reversible hydration of carbon dioxide (CO2) to hydrogen carbonate (HCO3–) and proton (H+). Therefore, they play key roles in diverse processes, such as physiological pH control, gas balance and calcification [Citation2]. At least 15 isoforms of CA have been identified in vertebrates with different physiological and pathological roles Citation3-5. Among CA isoforms of the cytosolic ones (CA-I, CA-II, CA-III, CA-VII) and mitochondria (CA-V), CA-I is found together with CA-II in erythrocytes. CA-II is the most widely distributed CA in the eye, kidney, central nervous system and inner ear Citation3Citation6-9.
The activity of CA isozymes in human erythrocytes has been shown to vary considerably under certain pathological and physiological conditions. Moreover, changes in CA activity have been associated with some metabolic diseases such as, diabetes mellitus and hypertension Citation9-11. The inhibition of CA has been shown to impair H+ secretion into the proximal small intestinal lumen, thereby decreasing bicarbonate resorption. In addition, the inhibition of CA leads to decreased acidification of urine, the production of alkaline urine and eventually metabolic acidosis [Citation12].
Ethanol is a widely consumed as sedative-hypnotic drug throughout the world [Citation13]. The interaction of the ethanol with intracellular signal transduction pathways has been linked to disturbances in cell proliferation and survival [Citation14]. Such perturbations may play a role in the pathogenesis of certain disorders of tissue and organ function associated with acute and chronic alcohol exposures, including neuronal degeneration, cirrhosis, myopathy, and cardiomyopathy. Also, the ethanol intake may lead to oxidative damage in several tissues including erythrocytes and plasma Citation15-18.
Although oxidant effects of the ethanol in erythrocytes are known, its effects on erythrocyte CA isozymes have not been studied yet. Therefore, in this in vitro and in vivo study we aimed to investigate the effects of the ethanol consumption on erythrocyte CA-I and CA-II activities.
Materials and methods
Chemicals
Protein assay reagents, Sepharose-4B and chemicals for electrophoresis were obtained from Sigma-Aldrich Co. (Sigma-Aldrich Chemie GmbH Export Department Eschenstrasse 5, 82024 Taufkirchen, Germany). Para-aminobenzene sulfonamide and L-tyrosine were from E. Merck (Merck KGaA Frankfurter strasse 250, D-64293 Darmstadt Germany). All other chemical substances used were analytical grade and obtained from either Sigma-Aldrich or E. Merck.
Purification of HCA-I and HCA-II by affinity chromatography
The haemolysate preparation and Sepharose 4B-L-tyrosine-sulfanilamide affinity chromatography were performed according to the our previously published method [Citation5,Citation9]. Purified CA enzyme was dialyzed for 24 h against 0.05 M Tris-SO4/1 mM 2-mercaptoethanol (pH 7.4). All procedures were performed at 4°C [Citation19]. Protein concentrations in the column effluents were determined at 280 nm spectrophotometrically.
In vitro studies
The effect of increasing concentrations of the ethanol on human CA isozyme activity was determined colorimetrically using hydratase activity. Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson (1976). CO2-Hydratase activity was calculated by using the equation , where t0 and t1 are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively, and this was used to express enzyme unit (EU). CO2-hydratase activity of enzyme was determined at room temperature in a veronal buffer (pH: 8.6) with Bromo Timol Blue as indicator and satured carbon dioxide solution as substrate in a final volume of 4.2 ml. The time (in seconds) taken for the solution to change from blue to yellow was measured.
In vivo studies
Eight adult Sprague-Dawley rats (200–250 g) were selected for intraperitoneally administration of the ethanol (2 mL.kg− 1) using animal protocols laid down by institutional guidelines. Blood samples (0.5 mL) were taken from each rat prior to the ethanol administration as well as at 1 h, 3 h and 5 h intervals thereafter. They were placed into test tubes containing EDTA (2 mM) and subjected to centrifugation at 2500 × g for 15 min at 4°C (HERMLE Z383K). The erythrocyte pellet was washed three times with cold 0.16 M KCl and the supernatant discarded. One volume of erythrocyte pellet was suspended in five volumes of ice water to give an erythrocyte haemolysate. Carbonic anhydrase has hydratase activity in vivo. Hence, in our in vivo studies, we preferred the hydratase activity of carbonic anhydrase. It was determined as colorimetrically by using the CO2-hydratase method [Citation9,Citation19].
Protein determination
Quantitative protein determination was done by absorbance measurement at 595 nm according to Bradford, with bovine serum albumin as standard [Citation20].
Haemoglobin determination
Haemoglobin (Hb) concentration in haemolysate was determined by cyanmethaemoglobin method described by Beydemir et al. [Citation21].
SDS polyacrylamide gel electrophoresis
Discontinuous polyacrylamide gel electrophoresis was performed under denaturing conditions after HCA-I and HCA-II purification according to the Laemmli's method [Citation21]. For HCA-I, 40 mg samples were applied to the electrophoresis medium in line 1, For HCA-II, 20 mg samples were applied to the electrophoresis medium in line 2, respectively. Gel was stained overnight in 0.1% Coommassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then distained by frequently changing the same solvent, without dye. The electrophoretic pattern was photographed (see ).
Statistical analyses
Data were expressed as the Mean ± S.D. (standard deviation). Statistical analysis comprised significance testing of the difference between means (control vs test) using a two-tailed Student's t-test at the levels: 0.05, 0.01 and 0.001.
Results and discussion
The ethanol content of various beverages varies between 2.5% (beer) and 55% (strong spirit). As well as some beneficial effects, the ethanol exerts serious side-effects in the central nervous system (CNS), liver and other tissues. It is known that the ethanol increases the generation of reactive oxygen species and leads to oxidative damage in erythrocytes [Citation24,Citation25]. In fact, the ethanol causes several haemolytic disorders due to both direct and indirect effects [Citation13]. Additionally, the ethanol metabolite acetaldehyde inside of the erythrocyte has the ability to generate free radical species and to cause deleterious effects on erythrocytes [Citation26]. Although several effects of the ethanol and its metabolites on erythrocytes have been reported previously, there has been no report to analyse its effects on erythrocyte CA enzymes. Given the importance of CA in pH regulation in most tissues, the effects of increasing concentration of the ethanol on human erythrocyte HCA-I and HCA-II isozymes were undertaken in this study
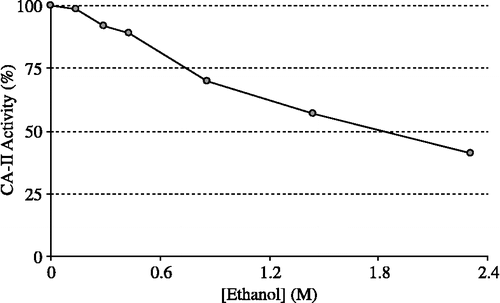
HCA-I and HCA-II were purified by Sepharose 4B-L-tyrosine-sulfanylamide affinity chromatography (). As can be seen in , the purity of both isoenzymes was confirmed by SDS-PAGE. The overall purification, HCA-I and HCA-II isozymes were obtained with a yield of 55.78 and 60.03, specific activities of 4311.8 and 13515.4 U/mg proteins respectively. Also, these isoenzymes were purified 600.9 and 1883.4-fold, respectively (). The range of the ethanol concentrations used was considered adequate to show enzyme inhibition effects. IC50 was 1.83 M for HCA-I (r2:0.99273) and 2.09 M for HCA-II (r2: 0.9749). In this regard, it was evident from in vitro studies that HCA-I and HCA-II were inhibited by the ethanol at 0.14–4.61 mM concentrations ( and ).
Table I. Purification scheme of HCA-I and HCA-II from human erythrocytes by Sepharose-4B-L-tyrosine-sulfanilamide affinity chromatography (HCA: Human carbonic anhydrase). Experimental analysis were performed at +4°C. CA enzyme activity was determined colorimetrically using CO2-hydration method of Wilbur and Anderson. The enzyme unit (EU) was calculated by using the Equation (to-tc/tc) where to and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
Figure 2 Effect of the ethanol at different concentrations on human erythrocyte CA-I activity (CA-I: Carbonic anhydrase isoform-I).
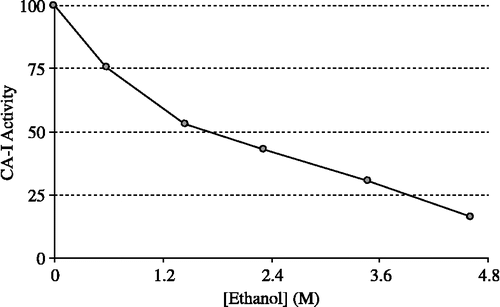
Figure 3 Effect of the ethanol at different concentrations on human erythrocyte CA-II activity (CA-II: Carbonic anhydrase isoform-II).
After the ethanol intake, blood level rises to a peak within 30–90 min [Citation27]. The half-life of the ethanol in plasma is highly variable, but a single dose of the ethanol will be disposed of for 6–8 h at all or even more [Citation27]. In this study, it was demonstrated that CA enzyme activities were inhibited 1 h after the ethanol administration and inhibition was continued significantly for 3 h in rats (). These results suggest a correlation between plasma peak level of the ethanol and maximal inhibition of CA enzyme activity, and between the plasma half-life of the ethanol and continuity of inhibition. Maximal inhibition of rat erythrocyte CA enzyme activity by 2 mL.kg− 1 the ethanol was within 3 hour (p < 0.01; ) after intraperitoneally the ethanol administration. Hence there is accordance between in vivo and in vitro inhibitory effect of the ethanol on CA activity. Moreover, the results of our study show that the ethanol itself is a highly potent inhibitor of erythrocyte CA isoenzyme both in in vivo and in vitro conditions.
Table II. The in vivo effects of dosage of ethanol (2 mL.kg−1 body weight) on rat red blood cell CA activity (CA: Carbonic anhydrase, n: 8).
The inhibition of CA is known to result in metabolic acidosis and may be is an ethological factor of metabolic acidosis occurred after the ethanol consumption which reported with several studies [Citation28,Citation29]. Acutely the ethanol impairs acid-base regulation, and acidosis seen in alcohol-intoxicated patients. Although the pathophysiology is complex, we think that one of the reason of metabolic acidosis due to the ethanol may be is the CA inhibition in erythrocytes. But the complexity of pathogenesis of acidosis in the ethanol intoxication requires further studies to rule out pathophysiology. In alcohol-intoxicated patients due to vomiting with resulting hypovolemia, saline and glucose solutions are administered to regulate electrolyte and acid-base disturbances. Probably in the treatment of alcohol intoxication CA enzyme will be one of the study subjects.
Conclusion
Since carbonic anhydrase produces and uses protons and bicarbonate ions, it plays a key role in the regulation of pH and fluid balance in different parts of human body. Especially, in stomach lining it plays a role in secreting acid, while the same enzyme helps to make pancreatic juices alkaline and saliva neutral. The transport of the protons and bicarbonate ions produced in kidney and eyes influence the water content of the cells at these locations. Thus carbonic anhydrase isozymes perform different functions at their specific locations. On the other hand, their absence or malfunction can lead to diseased states, ranging from the loss of acid production in the stomach to kidney failure.
In conclusion, the ethanol showed in vitro and in vivo inhibitory effects on erythrocyte CA isozymes activities. The CA inhibitors can cause of some side effects mentioned above. In this circumstance, the usage of ethanol in a patient with metabolic acidosis or the other diseases may cause serious side effects and worsen the clinical course. For this reason, the ethanol must be carefully used and their dosages should be very well ordered to decrease the side effects. But further studies are required to definitively establish its role on enzymes.
References
- Jones RP, Greenfield PF. Enzyme Microb Tech 1982; 4: 210–223
- Beydemir Ş, Çiftçi M, Küfrevioğlu ÖI, Buyukuroglu ME. Biol pharm Bull 2002; 25: 966–696
- Casini A, Scozzafava A, Mincione F, Menabuoni L, Starnotti M, Supuran CT. Bioorg Med Chem Lett 2003; 13: 2867–2873
- Hilvo M, Tolvanen M, Clark A, Shen BR, Shah GN, Waheed A, Halmi P, Hanninen M, Hamalainen JM, Vihinen M, Sly WS, Parkkila S. Biochem J Immediate publication 2005.
- Beydemir Ş, Gülçin I. J Enzyme inhib Med Chem 2004; 19: 193–197
- Parkkila AK, Scarim AL, Parkkila S, Waheed A, Corbett JA, Sly WS. J Biol Chem 1998; 273: 24620–24623
- Mori K, Ogawa Y, Ebihara K, Tamura N, Tashiro K, Kuwahara T, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, Nakao K. J Boil Chem 1999; 274: 15701–15705
- Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Bioorg Med Chem 2001; 9: 703–714
- Gülçin I, Beydemir Ş, Buyukokuroğlu ME. Biol Pharm Bull 2004; 27: 613–616
- Parui R, Gambir KK, Mehrotra PP. Biochem Internet 1991; 23: 779–789
- Parui R, Gambir KK, Cruz I. Biochem Internet 1992; 26: 809–820
- Hannedoeche T, Lazaro M, Delgado AG, Hosten AO. Clin Sci 1991; 81: 457–464
- Beydemir S, Gülçin I. J Anim Vet Adv 2003; 2: 143–146
- Lioyd MD, Thiyagarajan N, Ho YT, Woo LWL, Sutcliffe OB, Purohit A, Reed MJ, Acharya KR, Potter BVL. Biochemistry 2005; 44: 6858–6866
- Lee NM, Becker CE. Alcohol. Basic and Clinical Pharmacology. USA: Appleton and Large, BG Katzung, 1989; 278–286
- Luo J, Miller MW. Brain Res Rev 1998; 27: 157–167
- Hannigan JH, Armant DR. Semin Neonatol 2000; 5: 243–254
- Rubina R, Harrisonb R, Chenb XF, Corzitotto J, Hoek JB, Hallak H. Biochem Pharmacol 2004; 68: 2009–2017
- Bülbül M, Hisar O, Beydemir Ş, Ciftci M, Kufreevioglu IÖ. J Enzyme Inhib Med Chem 2003; 18: 371–375
- Braford MM. Anal Biochem 1976; 72: 248–251
- Beydenur Ş, Gülçin I, Küfrevioğlu Öİ, Çiftçi M. Pol J Pharmacol 2003; 55: 787–792
- Laemmli DK. Nature 1970; 227: 680–683
- Rang HP, Dale MM, Ritter JM. Pharmacology. Churchill Livingstone, China 1999; 623–628
- Sözmen EY, Tanyalcin T, Onat T, Kutay F, Erlacin S. Eur J Clin Chem Clin Biochem 1994; 32: 741–744
- Soszynski M, Schuessler H. Int J Radiat Biol 1998; 73: 211–218
- Tyulina OV, Huentelman MJ, Prokopieva VD, Boldyrev AA, Johnson P. Biochim Biophys Acta 2001; 535: 69–77
- Laurence DR, Bennett PN, Brown MJ. Clinical pharmacology8th Edn,. Churchill Livingstone, Singapore 1997; 111
- Zehtabchi S, Sinert R, Baron BJ, Paladino L, Yadav K. Clin Toxicol 2006; 43: 161–166
- Sibai K, Eggimann P. Rev Med Suisse 2005; 1: 2106–2112