Abstract
2-Hydroxy-1-naphthaldehyde derived sulfonamides and their first row d-transition metal chelates [cobalt (II), copper (II), nickel (II) and zinc (II)] have been synthesized and characterized. The nature of bonding and structure of all the compounds have been deduced from elemental analyses, infrared, 1H NMR, 13C NMR, mass spectrometry, electronic spectra, magnetic susceptibility and conductivity measurements. An octahedral geometry has been suggested for all the complexes. The metal complexes were screened for their antibacterial and antifungal activities on different species of pathogenic bacteria and fungi and their biopotency has been discussed. The results of these studies revealed that all compounds showed moderate to significant antibacterial activity against all bacterial strains and good antifungal activity against various fungal strains. In-vitro cytotoxic properties of all the compounds against Artemia salina was also studies by brine shrimp bioassay.
Introduction
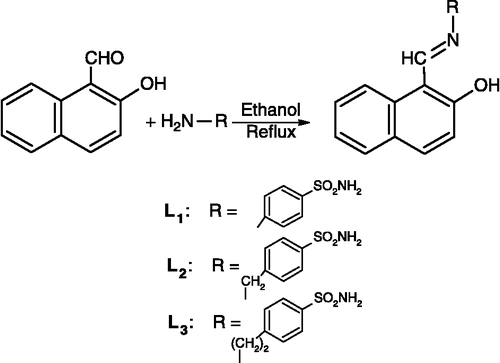
Sulfonamides are well-renowned for their antibacterial Citation1-3, antitumor [Citation4], diuretic [Citation5], anti-carbonic anhydrase [Citation6,Citation7], hypoglycaemic [Citation8], anti-thyroid [Citation9] and protease inhibitor [Citation10] activities. Many drugs possess modified pharmacological and toxicological potentials when administered in the form of metallic compounds. The most widely studied metallic ions in this respect are cobalt (II), copper (II), nickel (II) and zinc (II) because they form low molecular weight complexes which are proved to be more beneficial against several diseases Citation11-14. Sulfonamides have been known to attract much attention into this promising area of metal based sulfa drugs. It was primarily inspired by the successful introduction of metal based compounds of sulfadiazine to stop and/or heal bacterial infections Citation15-17. Biological properties of the metal complexes exclusively depend on the ease of cleaving the bond between the metal ion and the ligand. It is therefore, vital to understand coordination behaviour and relationship of the metals and the ligands in biological systems. In view of the versatile biological chemistry of sulfonamides and to identify the coordination properties we have consequently, activated a program Citation18-28, in synthesizing and designing various metal based sulphonamides and look into their structural and biological behaviour. In the same continuation, we herein describe the preparation, and characterization of Co(II), Cu(II) Ni(II) and Zn (II) complexes with 2-hydroxy-1-naphthaldehyde derived sulfonamides, 4-[(2-hydroxynaphthalen-1-yl)methyleneamino] benzenesulfonamide, 4-[{(2-hydroxynaphthalen-1-yl)methyleneamino}methyl]benzenesulfonamide, and 4-[2-{(2-hydroxynaphthalen-1-yl)methyleneamino}ethyl] benzenesulfonamide. Also, in-vitro antibacterial, antifungal and cytotoxic properties of these compounds have been evaluated and reported in the present paper.
Materials and methods
All reagents and solvents used were of analytical grades; Elemental analyses were carried out with a LECO-CHNS-9320 model. 1H and 13C-NMR spectra of compounds were recorded with a Bruker Spectrospin Avance DPX-400 using TMS as internal standard and DMSO d6 as solvent. Infrared spectra of the compounds were recorded on a Philips Analytical PU 9800 FTIR spectrophotometer. The melting points were determined with a Gallenkamp melting point apparatus. In vitro antibacterial, antifungal and cytotoxic properties were studied at HEJ Research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Pakistan.
Synthesis of ligands
Synthesis of 4-[(2-hydroxynaphthalen-1-yl)methyleneamino] benzenesulfonamide (L1)
To an ethanolic (30 ml) solution of sulfanilamide (1.21 g, 0.007 moles), 2-hydroxy-1-naphthaldehyde (1.21 g, 0.007 moles) in ethanol (15 ml) was added with stirring. The solution was refluxed for 2 h. The precipitates thus formed during refluxing, were cooled to room temperature and collected by suction filtration. Washing thoroughly with ethanol (2 × 10 ml), afforded TLC pure product (1.90 g, 83% yield).
The same method was applied to prepare ligands (L2) and (L3).
Physical measurements, analytical estimations and spectral properties of the ligands and zinc (II) complexes
4-[(2-hydroxynaphthalen-1-yl)methyleneamino] benzenesulfonamide (L1)
Yield 83%; m.p. 280–82°C; IR (KBr, cm− 1): 3392 (NH2), 3315 (OH), 1597 (HC = N), 1345, 1110 (S = O), 956 (S–N), 841 (C–S); 1H NMR (DMSO-d6, δ, ppm): 7.20 (s, 2H, SO2NH2), 7.50–7.85 (m, 4H, N-Ph), 7.90–8.25 (m, 6H, naphthalene), 9.15 (s, 1H, azomethine), 10.85 (s, 1H, OH); Anal. Calcd. for C17H14N2O3S (326.38): C, 62.56; H, 4.32; N, 8.58; Found: C, 62.75; H, 4.42; N, 8.53%; Mass spectrum (ESI) [M]+ = 325.9. 13C NMR (δ, ppm): 138.2 (C1-phenyl), 128.6 (C2,C6-phenyl), 122.6 (C3,C5-phenyl), 156.4 (C4-phenyl), 160.0 (C = N, azomethine), 108.5 (C1-naphthalene), 158.8 (C2-naphthalene), 118.4 (C3-naphthalene), 132.4 (C4-naphthalene), 126.8–135.1 (C5,C6,C7,C8,C9,C10-naphthalene); 1H NMR of Zn (II) complex (DMSO-d6, δ, ppm): 7.55 (s, 2H, SO2NH2), 7.90–8.15 (m, 4H, N-Ph), 8.20–8.55 (m, 6H, naphthalene), 9.45 (s, 1H, azomethine); 13C NMR of Zn (II) complex (δ, ppm): 138.2 (C1-phenyl), 128.6 (C2,C6-phenyl), 122.6 (C3,C5-phenyl), 165.2 (C4-phenyl), 172.3 (C = N, azomethine), 114.6 (C1-naphthalene), 170.1 (C2-naphthalene), 122.3 (C3-naphthalene), 126.8–135.1 (C4,C5,C6,C7,C8,C9,C10-naphthalene).
4-[{(2-hydroxynaphthalen-1-yl)methyleneamino}methyl]benzenesulfonamide (L2)
Yield 81%; m.p. 240–42°C; IR (KBr, cm− 1): 3392 (NH2), 3315 (OH), 1592 (HC = N), 1345, 1110 (S = O), 956 (S–N), 841 (C–S); 1H NMR (DMSO-d6, δ, ppm): 4.63 (s, 2H, CH2), 7.20 (s, 2H, SO2NH2), 7.50–7.85 (m, 4H, N-Ph), 7.90–8.25 (m, 6H, naphthalene), 9.00 (s, 1H, azomethine), 10.85 (s, 1H, OH); 13C NMR (δ, ppm): 136.7 (C1-phenyl), 127.2 (C2,C6-phenyl), 129.3 (C3,C5-phenyl), 142.1 (C4-phenyl), 64.4 (CH2), 160.8 (C = N, azomethine), 114.6 (C1-naphthalene), 158.8 (C2-naphthalene), 118.4 (C3-naphthalene), 132.4 (C4-naphthalene), 126.8–135.1 (C5,C6,C7,C8,C9,C10-naphthalene); Anal. Calcd. for C18H16N2O3S (340.40): C, 63.51; H, 4.74; N, 8.23; Found: C, 63.55; H, 4.65; N, 8.20%. Mass spectrum (ESI) [M]+ = 341.15. 1H NMR of Zn (II) complex (DMSO-d6, δ, ppm): 4.95 (s, 2H, CH2), 7.55 (s, 2H, SO2NH2), 7.90–8.15 (m, 4H, N-Ph), 8.20–8.55 (m, 6H, naphthalene), 9.40 (s, 1H, azomethine); 13C NMR of Zn (II) complex (δ, ppm): 136.7 (C1-phenyl), 127.2 (C2,C6-phenyl), 129.3 (C3,C5-phenyl), 142.1 (C4-phenyl), 70.1 (CH2), 171.3 (C = N, azomethine), 119.8 (C1-naphthalene), 169.2 (C2-naphthalene), 121.2 (C3-naphthalene), 126.8-135.1 (C4,C5,C6,C7,C8,C9,C10-naphthalene).
4-[2-{(2-hydroxynaphthalen-1-yl)methyleneamino}ethyl] benzenesulfonamide (L3)
Yield 80%; m.p. 197–98°C; IR (KBr, cm− 1): 3392 (NH2), 3315 (OH), 1591 (HC = N), 1345, 1110 (S = O), 956 (S–N), 841 (C–S); 1H NMR (DMSO-d6, δ, ppm): 2.91 (t, 2H, CH2), 3.88 (t, 2H, CH2), 7.20 (s, 2H, SO2NH2), 7.50–7.85 (m, 4H, N-Ph), 7.90–8.25 (m, 6H, naphthalene), 9.00 (s, 1H, azomethine), 10.85 (s, 1H, OH); 13C NMR (δ, ppm): 136.9 (C1-phenyl), 127.2 (C2,C6-phenyl), 128.0 (C3,C5-phenyl), 142.6 (C4-phenyl), 37.5 (CH2-C4), 61.3 (CH2–N = ) 160.8 (C = N, azomethine), 114.6 (C1-naphthalene), 158.8 (C2-naphthalene), 118.4 (C3-naphthalene), 132.4 (C4-naphthalene), 126.8–135.1 (C5,C6,C7,C8,C9,C10-naphthalene); Anal. Calcd. for C19H18N2O3S (354.43): C, 64.39; H, 5.12; N, 7.90; Found: C, 64.45; H, 5.22; N, 7.83%. Mass spectrum (ESI) [M]+ = 354.8. 1H NMR of Zn (II) complex (DMSO-d6, δ, ppm): 3.15 (t, 2H, CH2), 4.20 (t, 2H, CH2), 7.55 (s, 2H, SO2NH2), 7.90–8.15 (m, 4H, N-Ph), 8.20–8.55 (m, 6H, naphthalene), 9.40 (s, 1H, azomethine); 13C NMR of Zn (II) complex (δ, ppm): 136.9 (C1-phenyl), 127.2 (C2,C6-phenyl), 128.0 (C3,C5-phenyl), 142.6 (C4-phenyl), 37.5 (CH2-C4), 65.5 (CH2–N = ), 171.3 (C = N, azomethine), 119.8 (C1-naphthalene), 169.2 (C2-naphthalene), 121.2 (C3-naphthalene), 126.8–135.1 (C4,C5,C6,C7,C8,C9,C10-naphthalene).
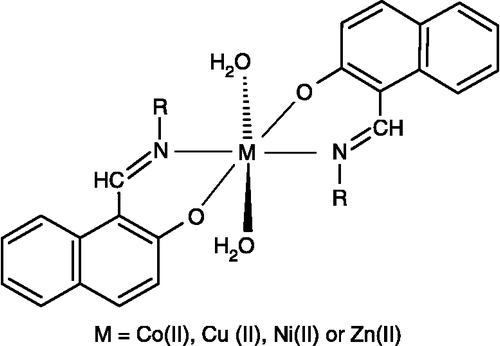
Synthesis of metal (II) complexes
Synthesis of Co(II) complex with 4-[(2-hydroxynaphthalen-1-yl)methyleneamino] benzenesulfonamide [Co(L1-H)2(H2O)2] (1)
To a hot magnetically stirred dioxane (10 ml) solution of 4-[(2-hydroxynaphthalen-1-yl)methyleneamino] benzenesulfonamide (L1) (0.65 g, 0.002 moles), an aqueous solution (15 ml) of Co (II) Cl2.6H2O (0.24 g, 0.001 moles) was added. Then buffer solution (pH = 10, 2 ml) was added in it to maintain the pH of the reaction mixture. The mixture was then refluxed for 1 h. The precipitates formed during refluxing, were cooled to room temperature, collected by suction filtration and washed with small amount of dioxane (1 × 5 ml), ether (2 × 10 ml) and dried. Unfortunately, only microcrystalline powder could be obtained, which was impossible to be used for X-ray structural determinations.
The same method was used for the preparation of all other complexes (2)-(12).
Biological activity
Antibacterial bioassay (in-vitro)
All the synthesized compounds (L1)-(L3) and metal (II) complexes (1)-(12) were screened in-vitro for their antibacterial activity against four Gram-negative (E. coli, S. flexenari, P. aeruginosa, S. typhi) and two Gram-positive (S. aureus, B. subtilis) bacterial strains by the agar-well diffusion method [Citation29,Citation30]. The wells (6 mm in diameter) were dug in the media with the help of a sterile metallic borer with centers at least 24 mm apart. Two to eight hours old bacterial inocula containing approximately 104–106 colony-forming units (CFU/ml) were spread on the surface of the nutrient agar with the help of a sterile cotton swab. The recommended concentration of the test sample (1 mg/ml in DMSO) was introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, imipenum, served as negative and positive controls, respectively. The plates were incubated immediately at 37°C for 24 h. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). In order to clarify any participating role of DMSO in the biological screening, separate studies were carried out with the solutions alone of DMSO and they showed no activity against any bacterial strains.
Antifungal activity (in-vitro)
Antifungal activities of all compounds were studied against six fungal cultures. Sabouraud dextrose agar (oxoid, Hampshire, England) was seeded with 105 (cfu) ml− 1 fungal spore suspensions and transferred to petri plates. Discs soaked in 20 ml (200 μg/ml in DMSO) of all compounds were placed at different positions on the agar surface. The plates were incubated at 32°C for seven days. The results were recorded [Citation31] as % of inhibition and compared with standard drugs miconazole and amphotericin B.
Minimum inhibitory concentration (MIC)
Compounds containing high antibacterial activity (over 80%) were selected for minimum inhibitory concentration (MIC) studies. The minimum inhibitory concentration was determined using the disc diffusion technique by preparing discs containing 10, 25, 50 and 100 μg/ml of the compounds and applying the protocol [Citation32].
Cytotoxicity (in-vitro)
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm), filled with artificial seawater, which was prepared with commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the matter compartment was opened to ordinary light. After two days nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 ml of DMF. From this stock solutions 500, 50 and 5 μg/ml were transferred to 9 vials (three for each dilutions were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 ml of DMF only. The solvent was allowed to evaporate overnight. After two days, when shrimp larvae were ready, 1 ml of sea water and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with sea water to 5 ml per vial. After 24 h the number of survivors was counted. Data were analyzed by Finney computer program to determine the LD50 values [Citation33,Citation34].
Result and discussion
Chemistry, composition and characterization of the ligands
The sulfonamide derived ligands (L1)-(L3) were prepared as shown in Scheme . All ligands were only soluble in Dioxane, DMF and DMSO. The composition of the ligands is consistent with their microanalytical data.
Chemistry, composition and characterization of the metal (II) complexes
The metal (II) complexes (1)-(12) of the ligands (L1)-(L3) were prepared according to the following equations:
Physical measurements and Analytical data for complexes (1)-(12) is given in .
Table I. Physical measurements and analytical data of the metal (II) complexes.
Conductance and magnetic susceptibility measurements
The molar conductance values (in DMF) for complexes (1)-(12) fall within the range 82–91 Ω− 1 cm2 mol− 1, showing their non-electrolytic [Citation35] nature. The room temperature magnetic moment values of the complexes are given in . The observed magnetic moment (4.82–4.85 B.M.) is consistent with half-spin octahedral cobalt (II) complexes. The magnetic moment values (1.79–1.83 B.M.) measured for the copper (II) complexes lie in the range expected for a d9- system, which contain one unpaired electron with octahedral geometry [Citation36]. The measured values (3.30–3.32 B.M.) for the nickel (II) complexes suggest [Citation37] octahedral geometry for these complexes (Scheme ). The zinc (II) complexes were found to be diamagnetic as expected.
Table II. Analytical conductivity, magnetic and spectral data of metal (II) complexes.
IR spectra
The important IR spectral bands of the ligands and its metal complexes are given in experimental and in . All ligands contain various potential electron pair donor sites. In the IR spectra of the ligands a broad band observed at 3315 cm− 1 and a sharp band at 1591–1597 cm− 1 are assigned [Citation38] to the ν(OH) and (C = N) modes respectively. Evidence of the nitrogen bonding of the azomethine (C = N) group to the central metal atom stems from the shift of the ν(C = N) frequency to lower frequency by 25–35 cm− 1 (1562–1572 cm− 1) in all of its metal complexes. This is further supported by the appearance of the new bands at 438–444 cm− 1 due to the ν(M-N) band [Citation39].
The coordination through the hydroxyl oxygen is revealed by disappearance of the mode at 3315 cm− 1 and appearance of a new band at 1395 cm− 1 due to the C–O mode. This is further confirmed by the appearance of the new band at 525–540 cm− 1 due to ν(M-O) in the metal complexes. The bands in the ligand due to νasymm(SO2) and νsymm(SO2) appear at 1345 and 1110 cm− 1, respectively [Citation40]. These bands remain almost unchanged in the complexes, indicating that this group is not participating in coordination. This is supported by the unchanged ν(S–N) and ν(C–S) modes appearing at 956 and 841 cm− 1, respectively [Citation41,Citation42], in the ligands after complexation. All the other potential electron pair donor sites of the ligands do not participate in coordination as their IR frequencies remain almost unchanged after complexation.
1H NMR spectra
1H NMR spectra of the free ligands and their diamagnetic zinc (II) complexes were recorded in DMSO-d6. The 1H NMR spectral data along with the possible assignments is recorded in the experimental part. All the protons due to heteroaromatic/aromatic groups were found as to be in their expected region [Citation43]. The conclusions drawn from these studies lend further support to the mode of bonding discussed in their IR spectra. The coordination of the azomethine nitrogen is inferred by the downfield shifting of the –CH = N– proton signal from 9.00–9.15 ppm in the ligand to 9.40–9.45 ppm in the complexes. Hydroxyl proton at 10.85 ppm in the spectra of Zn (II) complexes of ligands (L1)-(L3) disappeared indicating deprotonation and coordination of the oxygen with the metal ion All other protons underwent downfield shifting by 0.25–0.35 ppm due to the increased conjugation [Citation44] and coordination with the metal atoms. Furthermore, the number of protons calculated from the integration curves, and those obtained from the values of the expected CHN analyses agree well with each other.
13C NMR spectra
13C NMR spectra of the free ligands and their diamagnetic zinc (II) complexes were also recorded in DMSO-d6. The 13C NMR spectral data along with the possible assignments is recorded in the experimental part. The carbons atoms due to heteroaromatic/aromatic groups were found as to be in their expected region [Citation43]. The conclusions drawn from these studies present further support to the mode of bonding discussed in their IR and 1H NMR spectra. Downfield shifting of the –CH = N– signal from 160.0–160.8 ppm in the ligands to 171.3–172.3 ppm in its metal (II) complexes revealed coordination of the azomethine nitrogen to the metal atom. All other carbons underwent downfield shifting by 0.35–11.0 ppm due to the increased conjugation and coordination with the metal atoms [Citation44]. Furthermore, the presences of the number of carbons agree well with the expected values.
Mass spectra
The mass spectral data is consistent with the formulations: C17H14N2O3S, 325.9 (calcd., 326.38): C18H16N2O3S, 341.15 (calcd., 340.40): C19H18N2O3S, 354.8 (calcd., 354.43) of the ligands. The base peak for (L1) was observed at m/e 245.9 for fragment [C17H12NO]+ and for (L2) and (L3) at 169.82 for fragment [C11H8NO]+ as these are expected to be the most stable fragments.
Electronic spectra
The Co(II) complexes exhibited well-resolved, low-energy bands at 7,295–7,405 cm− 1, 17,445–17,510 cm− 1 and a strong high-energy band at 20,505–20,640 cm− 1 () which are assigned [Citation45] to the transitions 4T1g(F) → 4T2g(F), 4T1g(F) → 4A2g(F) and 4T1g(F) → 4T2g(P) in an octahedral geometry [Citation46]. A high intensity band at 29,315–29,370 cm− 1 was assigned to the metal to ligand charge transfer. The magnetic susceptibility measurements for the solid Co (II) complexes are also indicative of three unpaired electrons per Co (II) ion suggesting [Citation47] consistency with their octahedral environment.
The electronic spectra of the Cu (II) complexes () showed two low-energy weak bands at 14,985–15,155 cm− 1 and 19,160–19,205 cm− 1 and a strong high-energy band at 30,355–30,385 cm− 1 and may be assigned to 2B1g → 2A1g and 2B1g → 2Eg transitions, respectively [Citation48]. The strong high-energy band, in turn, is assigned to metal → ligand charge transfer. Also, the magnetic moment values for the copper (II) are indicative of anti-ferromagnetic spin-spin interaction through molecular association indicative of their octahedral geometry [Citation49].
The electronic spectra of the Ni (II) complexes showed d-d bands in the region 10,395–10,440, 15,690–15,785 and 26,455–26,535 cm− 1. These are assigned [Citation50] to the transitions 3A2g(F) → 3T2g(F), 3A2g(F) → 3T1g(F) and 3A2g(F) → 3T2g(P), respectively, consistent with their well-defined octahedral configuration. The band at 29,870–29,995 cm− 1 was assigned to metal → ligand charge transfer. The magnetic measurements showed two unpaired electrons per Ni (II) ion suggesting [Citation51] also an octahedral geometry for the Ni (II) complexes. The electronic spectra of the Zn (II) complexes exhibited only a high-intensity band at 28,935–29,125 cm− 1 and are assigned [Citation52] to a ligand-metal charge transfer.
Biological activity
Antibacterial bioassay (in-vitro)
All compounds were tested against four Gram-negative (E. coli, S. flexenari, P. aeruginosa, S. typhi) and two Gram-positive (S. aureus, B. subtilis) bacterial strains () according to literature protocol [Citation29,Citation30]. The results were compared with those of the standard drug imipenum (). All ligands showed moderate to significant activity against all Gram-negative and Gram-positive bacterial strains except the activity of all compounds against strain (b) where no moderate to significant activity was observed. Compounds (1)–(12) exhibited overall a significant activity against E. coli, P. aeruginosa, S. typhi, S. aureus and B. subtilis. However a moderate activity was observed by compound (3), (6) against (a), (L1), (L2), (L3) against (c) and (d), (L1) against (f). Antibacterial activity is overall enhanced after complexation of the ligands (). However the Zinc (II) complexes of all the ligands were observed to be the most active against all species. It was interesting to note that methyl and ethyl carbon chain in the ligands and their respective metal chelates had an impact on the bactericidal activity. As the carbon chain increased in compounds (5)-(12) the bactericidal activity was increased as compared to the other compounds of the series (1)-(4) where there was no methyl or ethyl carbon chain present.
Table III. Antibacterial bioassay (concentration used 1 mg/ml of DMSO) of ligands and metal (II) complexes.
Antifungal bioassay (in-vitro)
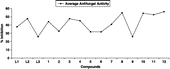
The antifungal screening of all compounds was carried out against T. longifusus, C. albican, A. flavus, M. canis, F. solani and C. glaberate fungal strains () according to the literature protocol [Citation31]. All synthesized compounds showed good antifungal activity against different fungal strains. Compound (10) and (11) showed good antifungal activity against all the fungal strains. The results of inhibition were compared with the results of inhibition of standard drugs miconazole and amphotericin B and individual synthesized compounds were also compared (). Effect of metal complexation on antifungal activity of the ligands can be seen ().
Table IV. Antifungal bioassay (concentration used 200 μg/ml) of ligands and metal (II) complexes.
Minimum inhibitory concentration (MIC) for antibacterial activity
The preliminary antibacterial screening showed that compounds (1), (4), (8) and (12) were the most active ones (above 80%). These compounds were therefore, selected for antibacterial minimum inhibitory concentration (MIC) studies ().
Table V. Minimum inhibitory concentration (moles/ml of the selected compounds (1), (4), (8) and (12) against selected bacteria.
Cytotoxic bioassay (in-vitro)
All the synthesized compounds were screened for their cytotoxicity (brine shrimp bioassay) using the protocol of Meyer et al. [Citation33]. From the data recorded in , it is evident that three compounds, (2), (6) and (10) displayed potent cytotoxic activity against Artemia salina, while the other compounds were almost inactive for this assay. The compound (2) showed activity (LD50 = 5.358 × 10− 4 moles/ml), compound (6) showed activity (LD50 = 6.745 × 10− 4 moles/ml), compound (10) showed activity (LD50 = 4.898 × 10− 4 moles/ml) in the present series of compounds. It was interesting to note that only copper complexes showed potent cytotoxicity whereas the other metal complexes did not. This activity relationship may help to serve as a basis for future direction towards the development of certain cytotoxic agents for clinical applications.
Table VI. Brine shrimp bioassay data of the ligands (L1)-(L3) and their metal (II) complexes (1)-(12).
Conclusion
The enhancement of antibacterial/antifungal activity in ligands (L1)-(L3) upon chelation is rationalized on the basis of their structures and the mode of coordination/chelation. It has been suggested that chelation reduces the polarity of the metal ion Citation53-56 on partial sharing of its positive charge with the donor groups. The process of chelation increases the lipophilic nature of the metal atom, which in turn favors Citation57-59 its permeation through the lipoid layer of cell membrane of the micro-organism. It has also been suggested that some functional groups such as azomethine or heteroaromatics present in these compounds display [Citation60,Citation61] extensive biological activities that may be responsible for the increase of hydrophobic character and liposolubility of the molecules. It ultimately enhances activity of the compounds and the biological utilization ratio.
Acknowledgements
One of us (HAS) is grateful to Higher Education Commission (HEC), Government of Pakistan for the award to carry out this research. We are also indebted to HEJ research Institute of Chemistry, University of Karachi, Pakistan, for help in providing NMR and mass spectra and also the antibacterial and antifungal assays.
References
- Mandell GL, Petri WA. Pharmacological basis of therapeutics9th edn, JG Hardman, LE Limbird, PB Molinoff, RW Ruddon, AG Gilman. McGraw-Hill, New York 1966; 1057–1072
- Maren TH. Relations between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol 1976; 16: 309–327
- Domagk G. Chemotherapy of bacterial infections. Deut Med Wochensch 1935; 61–250
- Owa T, Nagasu T. Novel sulfonamide derivatives for the treatment of cancer. Exp Opin Ther Pat 2000; 10: 1725–1740
- Boyd AE. Sulfonylurea receptors, ion channels, and fruit flies. Diabetes 1988; 37: 847–850
- Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenz A, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of the transmembrane isozyme XIV with sulfonamides. Bioorg Med Chem Lett 2005; 15: 3828–3833
- Scozzafava A, Briganti F, Mincione G, Menabuoni L, Mincione F, Supuran CT. Carbonic Anhydrase Inhibitors: Synthesis of water-soluble, aminoacyl/ dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 1999; 42: 3690–3700
- Thornber CW. Isosterism and molecular modification in drug design. Chem Soc Rev 1979; 8: 563–580
- Ogden RC, Flexner CW. Protease inhibitors in AIDS therapy. Marcel Dekker, New York, U.S.A 2001
- Supuran CT, Scozzafava A, Mastrolorenzo A. Bacterial proteases: Current theraputic use and future prospects for the development of new antibiotics. Exp Opin Therap Pat 2000; 111: 221–259
- Brown DH, Lewis AJ, Smith WE, Teape JW. Anti-inflammatory effects of some copper complexes. J Med Chem 1980; 23: 729–734
- Williams DR. The metals of life. Van Nostrand Reinhold, London 1971
- Ruiz M, Perello L, Ortiz R, Castineiras A, Maichlemossmer C, Canton E. Synthesis, characterization, and crystal-structure Cu(Cinoxacinate)2·2H2O complex. A square-planar CuO4 chromophore, antibacterial studies. J Inorg Biochem 1995; 59: 801–810
- Castillo-Blum SE, Barba-Behrens N. Coordination chemistry of some biologically active ligands. Coord Chem Rev 2000; 196: 3–30
- Bult A. Metal ions in biological systems, H Sigel, A Sigel. M. Dekker, New York: and references cited therein 1983; 261–268
- Guo Z, Sadler PJ. Metals in Medicine. Angew Chem Int Ed Engl 1999; 38: 1512
- Yuan RX, Xiong RG, Chen ZF, Zhang P, Ju HX, Dai Z, Guo ZJ, Fun HK, You XZ. Crystal structure of zinc(II) 2-sulfanilamidopyrimidine: A widely used topical burn drug. J Chem Soc Dalton Trans 2001; 774
- Chohan ZH. Synthesis and biological properties of Cu(II) complexes with 1,10-disubstituted ferrocenes. Synth React Inorg Met-Org Chem 2004; 34: 833
- Chohan ZH, Supuran CT, Scozzafava A. Metalloantibiotics: Synthesis and antibacterial activity of cobalt(II), copper(II), nickel(II) and zinc(II) complexes of kefzol. J Enz Inhib Med Chem 2004; 19: 79
- Chohan ZH, Scozzafava A, Supuran CT. Synthesis of biologically active Co(II), Cu(II), Ni(II) and Zn(II) complexes of symmetrically 1,10-disubstituted ferrocene-derived compounds. Synth React Inorg Met-Org Chem 2003; 33: 241
- Chohan ZH, Shaikh AU, Naseer MM, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic properties of metal-based furanyl derived sulfonamides. J Enz Inhib Med Chem 2006; 21: 771
- Hassan MU, Chohan ZH, Andrea S, Supuran CT. Carbonic anhydrase inhibitors: Schiff's bases of aromatic and heterocyclic sulfonamides and their metal complexes. J Enz Inhib Med Chem 2004; 19: 263
- Chohan ZH, Shaikh AU, Naseer MM. Metal-based isatin-bearing sulfonamides: their synthesis. Characterization and biological properties. Appl Organomet Chem 2006; 20: 729–739
- Chohan ZH. Antibacterial copper(II) complexes of 1,1-symmetric ferrocene-derived Schiff-base ligands: Studies of the effect of anions on their antibacterial properties. Appl Organomet Chem 2002; 16: 17
- Hassan MU, Chohan ZH, Supuran CT. Antibacterial Zn(II) compounds of Schiff bases derived from some benzothiazoles. Main Group Metal Chemistry 2002; 25: 291
- Chohan ZH, Scozzafava A, Supuran CT. Zinc complexes of benzothiazole-derived Schiff-bases with antibacterial activity. J Enz Inhib Med Chem 2003; 18: 259
- Chohan ZH, Scozzafava A, Supuran CT. Unsymmetrically 1,10-disubstituted ferrocenes: Synthesis of Co(II), Cu(II), Ni(II) and Zn(II) chelates of ferrocenyl -1-thiadiazolo-10-tetrazole, -1-thiadiazolo-10-triazole and -1-tetrazolo-10-triazole with antimicrobial properties. J Enz Inhib Med Chem 2002; 17: 261
- Puccetti L, Fosolis G, Daniela V, Chohan ZH, Andrea S, Supuran CT. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff's bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes in-vitro antibacterial, antifungal and cytotoxic properties of sulfonamide-derived Schiff's bases and their metal complexes. Bioorg Med Chem Lett 2005; 15: 3096
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. Harwood Academic Publishers, The Netherlands 2001; 16
- Chohan ZH. Synthesis of cobalt(II) and nickel(II) complexes of Ceclor (cefaclor) and preliminary experiments on their antibacterial character. Chem Pharm Bull 1991; 39: 1578
- Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. The Netherlands, Harwood Academic Publishers 2001; 22
- McLaughlin JL, Chang C-J, Smith DL. Studies in natural products chemistry, “Bench-Top” bioassays for the discovery of bioactive natural products: An update, structure and chemistry (part-B), Atta-ur-Rahman. Elsevier Science Publishers B.V., The Netherlands 1991; 9: 383
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica 1982; 45: 31
- Finney DJ. Probit analysis3rd edn. Cambridge University Press, Cambridge 1971
- Geary WJ. Use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev 1971; 7: 81
- Lever ABP, Lewis J, Nyholm RS. Square-planar bisethylenediamine-metal complexes. J Chem Soc 1963; 59: 2552
- Carlin RL. Transition metal chemistry2nd edn. Marcel Decker, New York 1965
- Bellamy LJ. The infrared spectra of complex molecules. John Wiley, New York 1971
- Ferrero JR. Low-frequency vibrations of inorganic and coordination compounds. John Wiley, New York 1971
- Burns GR. Metal complexes of thiocarbohydrazide. Inorg Chem 1968; 7: 277
- Maurya RC, Patel P. Synthesis, magnetic and special studies of some novel metal complexes of Cu (II), Ni (II), Co (II), Zn (II), Nd (III), Th (IV), and UO2 (VI) with schiff bases derived from sulfa drugs, viz., sulfanilamide/sulfamerazine and o-vanillin. Spectr Lett 1999; 32: 213
- Nakamoto K. Infrared spectra of inorganic and coordination compounds2nd edn. Wiley Interscience, New York 1970
- Simmons WW. The Sadtler handbook of protonNMRspectra. Sadtler Research Laboratories, Inc. 1978
- Pasto DJ. Organic structure determination. Prentice Hall International, London 1969
- Estes WE, Gavel DP, Hatfield WB, Hodgson DJ. Magnetic and structural characterization of dibromo- and dichlorobis (thiazole) copper (II). Inorg Chem 1978; 17: 1415
- Balhausen CJ. An introduction to ligand field. McGraw Hill, New York 1962
- Lever ABP. Inorganic electronic spectroscopy. Elsevier, Amsterdam 1984
- Chohan ZH, Kausar S. Biologically active complexes of nickel(II), copper(II) and zinc(II) with Schiff-base ligand derived from the reaction of 2-aminopyridine and pyrrol-2-carboxaldehyde—their synthesis and characterisation. Chem Pharm Bull 1992; 40: 2555
- Chohan ZH, Rauf A, Naseer MM, Somra MA, Supuran CT. Antibacterial, antifungal and cytotoxic properties of some sulfonamidederived chromones. J Enz Inhib Med Chem 2006; 21: 173–177
- Chohan ZH, Kausar S. Synthesis, structural and biological studies of nickel(II), copper(II) and zinc(II) chelates with tridentate Schiff bases having NNO and NNS donor systems. Chem Pharm Bull 1993; 41: 951
- Chohan ZH, Praveen M, Ghaffar A. Synthesis, characterisation and biological role of anions (nitrate, sulphate, oxalate and acetate) in Co(II), Cu(II) and Ni(II) metal chelates of some Schiff-base derived amino acids. Synth React Inorg Met-Org Chem 1998; 28: 1673
- Chohan ZH, Shaikh AU, Supuran CT. In-vitro Antibacterial, Antifungal and cytotoxic activity of cobalt (II), copper (II), nickel (II) and zinc (II) complexes with furanylmethyl- and thienylmethyl-dithiolenes: [1, 3-dithiole- 2-one and 1,3-dithiole-2-thione. J Enz Inhib Med Chem 2006; 21: 733
- Chohan ZH, Supuran CT, Scozzafava A. Metal binding and antibacterial activity of ciprofloxacin complexes. J Enz Inhib Med Chem 2005; 20: 303
- Chohan ZH, Farooq MA. Mixed ligand biologically active complexes of cobalt(II), copper(II), nickel(II) and zinc(II) with triazine derived NO and NS donor systems. J Chem Soc Pak 1995; 17: 14
- Chohan ZH, Sherazi SKA. Synthesis and spectroscopic studies of biologically active Co(II), Cu(II) and Ni(II) complexes of hydrazine derived Schiff-base ligands. J Chem Soc Pak 1997; 19: 196
- Rehman SU, Chohan ZH, Naz F, Supuran CT. In vitro antibacterial, antifungal and cytotoxic activities of some coumarines and their metal complexes. J Enz Inhib Med Chem 2005; 20: 333
- Chohan ZH, Supuran CT. Organometallic compounds with biologically active molecules: In-vitro antibacterial and antifungal activity of some 1,10-(dicarbohydrazono)ferrocenes and their Co (II), Cu (II), Ni (II) and Zn (II) complexes. Appl Organomet Chem 2005; 19: 1207
- Chohan ZH, Supuran CT. In-vitro antibacterial and cytotoxic activity of cobalt (II), copper (II), nickel (II) and zinc (II) complexes of the antibiotic drug cephalothin (keflin). J Enz Inhib Med Chem 2005; 20: 463
- Chohan ZH, Supuran CT, Scozzafava A. Metal binding and antibacterial activity of ciprofloxacin complexes. J Enz Inhib Med Chem 2005; 20: 303
- Chohan ZH, Supuran CT. Metalloantibiotics: Synthesis, characterization and in-vitro antibacterial studies on cobalt (II), copper (II), nickel (II) and zinc (II) complexes with cloxacillin. J Enz Inhib Med Chem 2006; 22: 69
- Chohan ZH. Antibacterial and antifungal ferrocene incorporated dithiothione and dithioketone compounds. Appl Organomet Chem 2006; 20: 112–116