Abstract
The synthetic, spectroscopic, and biological studies of Cu(II), Ni(II), Zn(II), Co(II), Mn(II), Fe(III) and Cr(III) complexes of N4-(7′-chloroquinoline-4′-ylamino)-N1-(2-hydroxy-benzylidene)thiosemicarbazone (HL) obtained by the reaction of N4-(7′-chloroquinolin-4′-ylamino)thiosemicarbazide with 2-hydroxybenzaldehyde. The structures of the complexes were determined on the basis of the elemental analyses, spectroscopic data (IR, electronic, 1H and 13C NMR and Mass spectra) along with magnetic susceptibility measurements, molar conductivity and thermogravimetric analyses. Electrical conductance measurement revealed the non-electrolytic nature of the complexes. The resulting colored products are mononuclear in nature. On the basis of the above studies, only one ligand was suggested to be coordinated to each metal atom by thione sulfur, azomethine nitrogen and phenolic oxygen to form mononuclear complexes in which the thiosemicarbazone behaves as a monobasic tridendate ligand. The ligand and its metal complexes were tested against Gram + ve bacteria (Staphylococcus aureus), Gram − ve bacteria (Escherichia coli), fungi (Candida albicans) and (Fusarium solani). The tested compounds exhibited significant activity.
Introduction
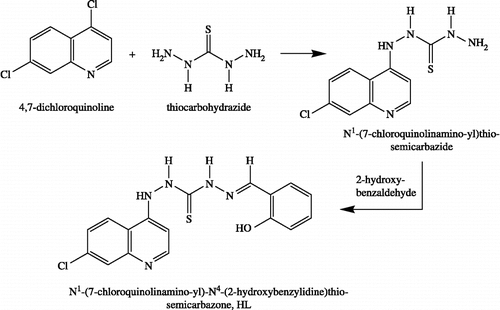
The synthesis and structural investigations of thiosemicarbazone and their metal complexes are of considerable centre of attention because of their potentially beneficial pharmacological properties and a wide variation in their modes of bonding and stereochemistry Citation1-3. Thiosemicarbazones that are most widely studied are sulfur and nitrogen consisting ligands [Citation4,Citation5]. Besides, thiosemicarbazones have emerged as an important sulfur containing ligands in the last two decades Citation6-9. The real impetus towards coordination chemistry is the wide range of biological properties depending on parent aldehyde or ketone including antitumour [Citation10,Citation11], antibacterial, and antifungal [Citation12,Citation13] as well as their physicochemical effects [Citation14,Citation15]. In addition of this, they have been screened for their medical properties because they possess some cytotoxic effect. They also stabilize uncommon oxidation states, generate a different coordination number in transition metal complexes in order to participate in various redox reactions [Citation16,Citation17]. It is well known that several metal ions enhance and modify the biological activities of thiosemicarbazones [Citation13]. Much attention has been drawn towards the chemistry of transition metals Citation18-22 in different coordination spheres. Due to different oxidation states of transition metals and their reactivity depends upon stability of oxidation states. In view of this ruthenium (III), thiosemicarbazones with nitrogen and sulphur as donor atoms have been found to be very efficient catalysts in the oxidation of alcohols and alkenes [Citation23]. With the growing interest of thiosemicarbazones Citation24-27, the present work was undertaken in order to investigate the ligational behaviour of the thiosemicarbazone derived from N4-(7′-chloroquinolin-4′-ylamino)thiosemicarbazide with 2-hydroxy-benzaldehyde () towards metal ions, Cu(II), Ni(II), Zn(II), Co(II), Mn(II), Fe(III) and Cr(III) as well as their biological activity in inhibiting the growth of some pathogenic bacteria.
Experimental
Materials
Reagent grade chemicals were used without further purification. CuCl2.2H2O, CoCl2.6H2O, NiCl2.6H2O, MnCl2.4H2O, ZnCl2, FeCl3.6H2O, CrCl3.6H2O and Li(OH).H2O were obtained from BDH. Hydrazine hydrate, 4,7-dichloroquinoline, and carbon disulphide, were either from BDH or Mercks solvents used were reagent grade.
Synthesis of the ligand
Synthesis of thiocarbohydrazide, (TCH), (I)
The preparation was carried out according to the previously reported method [Citation28,Citation29]. Carbon disulphide (5 mL) was added gradually to 20 mL hydrazine hydrate (90%). The reaction mixture was refluxed for 30 min. The reaction mixture was cooled in a water bath for further 30 min. and the formed yellow precipitate was filter off and washed several times with ethanol followed by diethyl ether. The white crystals were then re-crystallized from the least amount of water then dried over anhydrous CaCl2 and collected (yield 80%, m.p. 171°C,).
Synthesis of N4-(7′-chloroquinolin-4′-ylamino)thiosemicarbazide, (II)
A hot solution of 4,7-dichloroquinoline (2 g, 10 mmol dissolved in 20 mL ethanol) was added to thiocarbohydrazide (TCH) (I) (1 g, 10 mmol) dissolved in the least amount of ethanol-water mixture (10/5 v/v). The reaction mixture was left under stirring while it is hot for 30 min and then allowed to cool. The formed deep yellow crystals were filter off, washed several times with hot ethanol and finally dried over anhydrous CaCl2 for 24 hr. (yield 65%, m.p. 245°C) ().
Synthesis of N4-(7′-chloroquinolin-4′-ylamino)thiosemicarbazone, (HL)
The ligand HL was prepared by adding 2-hydroxybenzaldehyde (1.12 g., 10.1 mmol, dissolved in 10 mL absolute ethanol) to the thiocarbazide (II), (0.2 g, 10 mmol dissolved in 10 mL absolute ethanol). The reaction mixture was stirred thoroughly and refluxed for 2 h, golden orange crystals precipitated which were filtered and washed with few drops of ethanol (yield 85%, m.p. 265°C). The proposed chemical structure () of the ligand, HL, is known to be in good agreement with the ratios concluded from analytical data ().
Table I. Physicochemical parameters of ligand and it's complexes.
Synthesis of the metal complexes
A general method has been used for the preparation of the complexes using stoichimetric reaction of the metal salts and the corresponding Schiff base in presence of Li(OH) in molar ratio (M:L:Li(OH) = 1:1:1). Lithium hydroxide (0.1 g, 2.4 mmol) was dissolved in 5 mL methanol was added drop-wise to a stirred solution of the ligand (0.7 g. 2.4 mmol dissolved in 5 mL methanol). Metal salt solution (2.4 mmol dissolved in 10 mL methanol) was added gradually to the stirred solution of the lithium salt of the ligand. The precipitated solid complex was filtered off, washed with 50% (v/v) methanol-water mixture to remove any traces of the unreacted starting materials, then washed with diethyl ether and dried in vacuum desiccator over CaCl2. The obtained solid metal complexes and their colours are shown in . The complexes are stable solid, and some of them decomposed above 250°C without melting. They are insoluble in common organic solvents such as ethanol, methanol, chloroform and acetone. However, they are soluble in DMSO and DMF.
Physical measurements and analyses
Carbon, hydrogen, nitrogen and sulfur microanalyses were carried out at the analytical center of King Khalid University, Assir, Abha, Saudi Arabia using a Perkin Elmer 2400 Series Analyzer. Analyses of the metals followed decomposition of their complexes with concentrated nitric acid. The resultant solution was diluted with distilled water, filtered to remove the precipitated ligand. The solution was then neutralized with aqueous ammonia solution and the metal ions titrated with EDTA. Electronic spectra were recorded for the solutions of the ligand in ethanol, and for metal complexes as Nujol Mull on a Shimadzu UV-vis spectrophotometer model 1601 in the range 190-1100 nm. IR spectra were recorded as KBr discs using a FT-IR 4000 Perkin Elmer Spectrometer. The 1H NMR spectra were recorded at room temperature on Brucker WP 250 (250 MHz) spectrometer in DMSO-d6. The 13C NMR spectra were recorded at ambient temperature on Brucker 250 WM (62.9 MHz) spectrometer in DMSO-d6. Chemical shifts are given in ppm downfield from TMS. The halide content was then determined by titration using diphenyl carbazone as an indicator. Magnetic susceptibilities of the complexes were measured by the Gouy's method at room temperature using a model MK1 Johnson Matthey. Alpha products magnetic susceptibility balance. The effective magnetic moments were calculated using the relation (μeff = 2.828 (χm T)½ B.M. where χm is the molar susceptibility corrected using Pascal's constants for diamagnetism of all atoms in the compounds. The TG-DTA measurements were carried out on a Shimadzu thermo gravimetric analyzer in dry nitrogen atmosphere and a heating rate of 10°C/min using the TA-50 WS1 program. Mass spectra were recorded at 70 eV and 300°C on a MS 5988 Hewlett-Packard mass spectrometer. Conductivity measurements were measured in DMF solutions of the complexes (10− 3 M) using a model LBR, WTWD-812 Wilhelm Conductivity meter fitted with a model LTA 100 cell.
Pharmacology
The in-vitro evaluation of antimicrobial activity was performed according to the diffusion technique on solid media [Citation30]. Bacteria including Staphylococcus aureus, Escherichia coli were grown in nutrient broth at 37°C for 24 h. Candida albicans and Fusarium solani were grown in malt broth at 28°C for 48 h. Sterile (5 mm) diameter sensitivity discs were impregnated with different conc. of the ligand/complex (50 μg or 100 μg/mL) dissolved in DMSO. Discs of each tested compound were laid onto nutrient agar for bacteria or potato dextrose agar for fungi. Plates are surface spread with 0.2 mL of logarithmic phase bacteria or fungi cultures. A 0.5 mL spore suspension (108 spores/mL) for bacteria or for filamentous fungi was also spread onto potato dextrose agar plates. The plates were then incubated for 24 h at 37°C for bacteria and for 48 h at 28°C for fungi. Additionally antibiotic discs for cephalosporin and streptomycin were tested as positive control. The results were recorded by measuring the zones of growth inhibition surrounding the discs.
Results and discussion
Chemistry
The thiosemicarbazone Schiff base ligand, HL is expected to act as a heptadentate ligand, the possible coordination sites being quinoline nitrogen, phenolic oxygen, imine nitrogen, thio-keto sulfur and azomethine nitrogen. A study and comparison of the IR spectra of the ligand and its metal complexes imply that the Schiff base behave as monobasic tridendate ligands with phenolic-oxygen, azomethine-nitrogen, and the thio-keto sulfur as ONS ligand. The present thiosemicarbazone ligand exists as the thio-keto form since it has –NH–C = S thio amide group; although, in many instances, thiol form or equilibrium mixture of both forms has been observed in thiosemicarbazones. All the complexes are insoluble in common organic solvents such as ethanol, methanol, chloroform or acetone. However, they are soluble in DMSO and DMF. They are amorphous powder, stable at room temperature and do not show any decomposition on standing for two months. The molar conductance of the complexes in DMF lies in the range 18.5-11 Ω− 1 cm2 mol− 1(). The values are too low to account for any dissociation, therefore the complexes are considered to be nonelectrolytes [Citation31].
Infrared spectra
The binding mode of the ligand to metal ions was further elucidated by analysis of the IR spectra () of the ligand and its metal complexes formation. A study and comparison of infrared spectra of free ligand and its metal complexes, , imply that the Schiff base behaves as monobasic tridentate ligand and the metal ion is coordinated through the deprotonated oxygen atom of the phenolic, the nitrogen atom of the azomethine and the sulfur atom of thio-keto groups.
Table II. Characteristic IR bands (cm−1) of HL and its metal complexes.
The disappearance of the absorption bands associated with the stretching ν(O–H) of the phenolic groups (observed at 3530 cm− 1 in the free ligand) in all the spectra of the metal complexes, indicates a loss of phenolic proton on complexation and thus forming a metal-oxygen bond. The δ(COH)ip mode Citation32-34 which appeared at 1355 cm− 1 in the spectrum of the ligand, was not observed in the spectra of the complexes and thus supported the suggestion that the ligand coordinate to the metal through its deprotonated form. The presence of coordinated water was suggested by the very broad absorption centered around ∼3430 cm− 1 in the infrared spectra. Bands at ∼930 and ∼770 cm− 1 may be attributed to rocking and wagging modes of the coordinated water Citation32-35.
Furthermore, the band observed at ∼3270 cm− 1 region in the free ligand have been assigned to ν(NH) vibrations. Practically no effect on this frequency after complexation indicating the noninvolvement of this group in coordination with the metal ion. The most notable change in the ligand spectral features when coordinated to metal ion is the observed C = N red shift. The ν(C = N) band Citation32-34 at 1570 cm− 1 in the ligand spectrum exhibited a red shift of 50-40 cm− 1 in the spectra of the complexes. This finding may be taken as evidence for participation of the C = N group in coordination to the metal ions.
The intensity of the medium band at 1120 cm− 1 assigned for ν(N–N) in spectrum of the ligand, remains unchanged in all the spectra of the complexes, however, it shifted to the higher frequency Citation34-38. The strong band observed at 876 cm− 1 in the spectrum of HL, is mainly due to the stretching vibrations of (C = S), is shifted towards lower frequency and occurred at 850-820 cm− 1. in the corresponding spectra of the metal complexes indicating the coordination of the thio-keto sulphur to metal atom [Citation34].
The bands observed at 1586-l7400 cm− 1 assigned for ν(C = C) +ν(C = N) of the quinoline ring and at 577-462 and 478-700 cm− 1 due to in- and out-plane quinoline ring deformations, respectively, and that observed in the spectrum of the ligand at 827-1013 cm− 1 due to the quinoline ring breathing remain unchanged in frequency and band intensities revealing the noninvolvement of the quinolinic-nitrogen atom in the coordination with the metal ion.
The possibility of thione-thiol tautomerism (H–N–C = S), (C = N.SH) in the HL ligand has been ruled out for no band around 2700-2500 cm− 1, characteristic of thiol group is observed in the infrared absorption Citation32-34. The new bands observed in the regions 560-540, 460-445 and 365-355 cm− 1 are tentatively assigned to ν(M-O), ν(M-N) and ν(M-S) (metal-ligand) stretching bands, respectively [Citation34].
In conclusion, the infrared spectral studies suggest the monobasic tridentate nature of the ligand with ONS coordination sites. This was accounted for as the ligand contains phenolic OH, azomethine and ketonic-oxygen or thio-keto groups.
Magnetic moments and electronic spectral data of the metal complexes
The electronic spectra and magnetic moments of the metal complexes are listed in . Generally, in all spectra of metal complexes, the absorption bands due to π-π* and n-π* transitions that observed in the spectrum of the free ligand have shifted to lower frequencies due to the coordination of the ligand with metal ions.
Table III. Magnetic moment, electronic spectral data for HL and its metal complexes.
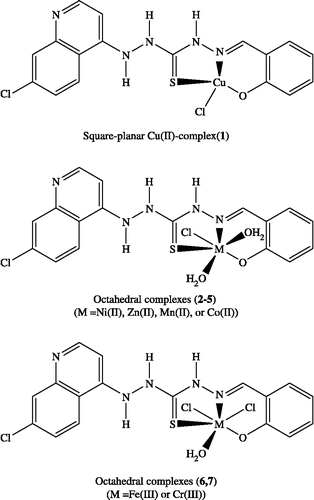
The spectra of the Cu(II) complex (1) () showed absorption bands at 240, 311, 325, and 348 nm attributed to ligand absorptions and a band at 665 nm is due to the d → d transition within the Cu(II) cation in square-planar geometry Citation35-40. The magnetic moments value, 1.78 B.M. reveals the presence of one unpaired electron and shows no copper-copper interaction. The square-planar geometry is achieved by the coordination of HL molecule as monobasic tridentate ligand, to the copper(II) ion. The shift of the d → d absorption band to lower energy than that expected for square-planar geometry, at 550 nm for square-planar N,N′-ethylenebis(salicylideneimine)-copper(II), Cu(acacen) [Citation39] may be due to the distortion of the square-planar geometry towards tetrahedral Citation35-39.
The electronic spectrum of the Ni(II) complex (2), , showed a main band at 768 nm and a shoulder at 670 nm. The main band may be due to the 3A1 g → 3T1 g electronic transition in an octahedral geometry. The 3A2 g → 3T1 g transition may be overlapped by the ligand transition, which appeared at 355 nm Citation35-39. The third transition due to 3A2 g → 3T2 g would be out of the range of the used spectrophotometer. The magnetic moment of the complex is 2.85 B.M. which agrees with the presence of Ni(II) ion in an octahedral geometry Citation36-39. This indicates that the Ni(II) ion coordinated to ONS sites in an octahedral geometry Citation36-39. The Ni(II) ion complete its six-coordination sphere by two water molecules and chloride ion.
Octahedral, tetrahedral and square-planar cobalt (II) complexes show magnetic moment between 4.7-5.2, 4.2-4.8 and 2.2-2.9 B.M., respectively [Citation25,Citation27]. The μeff value measured for the present Co(II) complex (4), (), is 5.2 B.M. which indicates that Co(II) ion is present in an octahedral geometry. The electronic spectrum of the complex showed two d-d transitions in the range 656 nm and shoulder at 477 nm due to the electronic transitions 4T1g → 4A2 g and 4T1g(F) → 4T1g(P), respectively, Citation37-39 indicating an octahedral configuration around Co(II) ion. The 4T2g(F) → 4T1g(F) electronic transition would be observed in near IR. This region is out of the range of our Uv-vis spectrophotometer.
The spectrum of the Mn(II) complex (5) showed a series of weak bands in the range 469-853 nm. These bands are both Laporte and spin-forbidden. However, due to instantaneous distortion of the octahedral structures around the metal cation, weak bands sometimes do appear Citation37-39. The magnetic moment of the complex is 5.39 B. M.
On the other hand, the electronic spectra of Fe(III) complex (6) showed broad bands 577 nm and at 722 nm. The former band may be due to the spin forbidden transition 6A1 g → 4T2 g, which may gain intensity as a result of the vibronic mechanism in the octahedral field around ferric ion. The second bands may be attributed to 6A1 → 4T1 transitions [Citation25]. In addition, a third absorption band with high intensity observed at 396 nm assigned for charge transfer transition. The magnetic moment of the complex is 5.9 B. M Citation36-39.
The spectrum of the Cr(III) complex (7) showed two broad bands at 592 and 396 nm besides the bands due to the ligand adsorptions. The observed bands at 592 and 396 nm may be due to 4A2 g → 4T2 g and 4A2 g → 4T1 g electronic transitions, respectively [Citation35,Citation39]. The 4A2 g → 4T1g(P) transition would be in the UV region and overlapped by ligand transitions. The expected value for μeff of a single Cr(III) ion in its octahedral complexes is 3.80 B.M. The spectrum of the diamagnetic Zn(II) complex (3) is dominated only by the ligand bands [Citation37]. Base on the above results, the structures in () are suggested for the metal complexes.
1H and 13C NMR
Coordination of thiosemicarbazone, HL, in the metal complexes are further confirmed by 1H and 13C NMR spectra [Citation40,Citation41] (). The 1H NMR spectrum of diamagnetic Zn(II) (3), showed the disappearance of the signal due to the proton of the phenolic OH group, appeared at δ 9.83 ppm in the ligand spectrum. This is attributed to its involvement in coordinating the zinc cation. Significant azomethine proton signal, due to CH = N, was observed at δ 7.52 ppm in ligand, and in complexes it has shown a change as a downfield shift and occurred at δ 8.22 ppm, indicating involvement of nitrogen in coordination. The proton peak of N–H group at δ 2.4 and 11.67 ppm remains the same in the ligand, and in the complexes it suggested that deprotonation do not occur and it has also shown keto form of the ligand. The signal due to the S–H (thiol) is not observed in the expected range 2-3 ppm [Citation34,Citation40] indicating that the ligand exist in the thione form. The multiplets as strong bands in region δ 7.7-8.24 ppm were assigned to aromatic and quinoline ring protons, which also shifted down field in the complexes.
Table IV. 1H and 13C NMR spectral data (δppm) of the ligand HL and its Zn(II) complex.
The 13C NMR spectra revealed the presence of expected number of signals corresponding to different types of carbon atoms present in the compounds. The spectrum of the ligand exhibit a strong band at δ 176 ppm and are assigned as C = S group [Citation41]. This band undergoes upfield shift of δ 7.2 ppm and occurs at δ 169.3 ppm. This has shown involvement of thione sulfur in coordination. The signal due to azomethine carbon occurred at δ 165.5 ppm as downfield peak, and on complexation it has shown shift to δ 161.3 ppm due to the resonance and also have given proof that nitrogen is involved in coordination. The O–C (C6) absorbs at δ 159.7 ppm in the ligand and on complexation and it has shown downfield shift to δ 162.6 ppm due to the resonance and also have given proof that nitrogen is involved in coordination. Due to the deshielding of the directly attached electronegative oxygen atom.
Mass spectra
The mass spectrum of the ligand, HL, showed its molecular ion at m/e 372 15%) which coincide with formula weight. The base peak observed at m/e 142 corresponded to the ion [C9H6N2]+. The metal complexes, melted or decomposed at relatively low temperature (250-300°C) and soluble in DMF and DMSO indicating the non-polymeric nature of the complexes which consequently confirmed the noninvolvement of nitrogen atoms of the quinoline moiety. Moreover, mass spectrum of Zn(II) complex, (3), which was chosen as representative example of this type of complexes, show a peak at m/e = 543 (8.5%) corresponding to the molecular ion, [C17H21N5Cl2O5SZn]+. The base peak appeared at m/e = 331 (100%) corresponded to the formation of a fragment [C16H14N2O2Zn]+. Metastable ion(s) is/are not observed [Citation34,Citation41].
Thermal analyses
Generally, there is less known about the thermal properties of transition metal complexes of thiocarbazones Citation42-45. In the present work, we report herein the thermal decomposition data of a representative complex, [(L)Zn(Cl)(OH2)2].2H2O, (3), that is, presented in . Analyses of thermogravimetric curve suggest that, the complex contain two molecules of lattice water, which is evident by loss in weight at 120-150°C. A second decomposition step was observed at 180-210°C, in which the complex loss two coordinated water molecules. After that, the complex loss one molecule HCl, one molecule HCN and NH-C-NH2 in one step at 250-350oC. There is no change up to ∼400°C after that the rest of the organic ligand began to decompose at ∼550°C. Finally, at ∼760°C, metal oxide, ZnO, formed Citation42-45.
Table V. Thermal analyses data for Zn(II) complex.
Antimicrobial activities results
A number of authors Citation3Citation46-49 were interested to investigate the biological and medicinal properties of transition metal complexes of thiosemicarbazones. Thomas and Parmeswaran [Citation46] studied the antitumour activities of Mn(II), Co(II), Ni(II), and Cu(II) chelates of anthracene-9-carboxaldehyde thiosemicarbazone. Murthy and Dharmaraja [Citation47] reported the cytotoxic activity of phenylglyoxal bis(thiosemicarbazone) against Ehrlich ascites carcinoma cells. These compounds were also screened for antimicrobial activity on Bsubtilis and E. coli. They inhibited the bacterial growth considerably.
The Schiff base, HL and its metal complexes reported were evaluated for antimicrobial activity against one strain Gram + ve bacteria (S. aureus) (a), Gram − ve bacteria (E. coli) (b), fungus (C. albicans), (c), fungus F. solani, (d). The results of the antimicrobial screening are presented in . Generally, the thio-keto Schiff base HL and its metal complexes were found to be biologically active. However, remarkable antimicrobial activity was shown for the metal complex, which is expected as it contains sulfur and nitrogen atoms. The results show, also, that all metal complexes exhibit antimicrobial activity to one or more strain and enhanced it comparing with the parent Schiff base.
Table VI. Antimicrobial activity of the Schiff base HL and its metal complexes.
It is known that chelation Citation25-28Citation50 tends to make the ligand act as a more powerful and potent bactericidal agent. A possible explanation for this increased activity upon chelation is that in the complex, the positive charge of the metal is partially shared with the donor atoms present on the ligand and there is an electron delocalization over the whole chelate ring. This, in turn, increase the lipophilic character of the metal chelate and favours its permeation through the lipoid layers of the bacterial membranes. Generally, it is suggested that the complexes deactivate various cellular enzymes, which play a vital role in various metabolic pathways of these microorganisms. Other factors such as solubility, conductivity and dipole moment, which affected by the presence of metal ions, may also be possible reasons for increasing the biological activity of the metal complexes as compared to the ligand from which they are derived.
References
- Mishra D, Naskar S, M GB, Chattopadhyay SK. Synthesis, spectroscopic and redox properties of some ruthenium(II) thiosemicarbazone complexes: structural description of four of these complexes. Inorgan Chim Acta 2006; 359: 585–592
- Casas JS, Garcia-Tasende MS, Sordo G. Main group metal complexes of semicarbazones and thiosemicarbazones, A structural review. Coord Chem Rev 2000; 209: 197–261
- Padhye' S, Kauffman GF. Transition metal complexes of semicarbazones and thiosemicarbazones. Coord Chem Rev 1985; 63: 127–160
- Pal I, Basuli F, Bhattacharya S. Thiosemicarbazone complexes of the platinum metals. A story of variable coordination modes. Proc Indian Acad Sci: Chem Sci 2002; 114: 255–268
- Belicchi Ferrari M, Capacchi S, Pelosi G, et al. Synthesis, structural characterization and biological activity of helicin thiosemicarbazone monohydrate and a copper(II) complex of salicylaldehyde thiosemicarbazone. Inorgan Chim Acta 1999; 286: 134–141
- Dutta S, Basuli F, Peng S-M, Lee G-H, Bhattacharya S. Synthesis, structure and redox properties of some thiosemicarbazone complexes of rhodium. New J Chem 2002; 26: 1607–1612
- El-Sawaf AK, West DX, El-Saied FA, El-Bahnasawy RM. Synthesis, magnetic and spectral studies of iron(III), cobalt(II, III), nickel(II), copper(II) and zinc(II) complexes of 4-formylantipyrine N(4)-antipyrinylthiosemicarbazone. Trans Metal Chem 1998; 23: 649–655
- Purohit S, Koley AP, Prasad LS, Manoharan PT, Ghosh S. Chemistry of molybdenum with hard-soft donor ligands.2.Molybdenum(VI),-(V), and -(IV) oxocomplexes with tridentate Schiff base ligands. Inorgan Chem 1989; 28: 3735–3742
- West DX, Swearingen K, Valde's-Martinez J, et al. Spectral and structural studies of iron(III), cobalt(II,III) and nickel(II) complexes of 2-pyridineformamide N(4)-methylthiosemicarbazone. Polyhedron 1999; 18: 2919–2929
- Afrasiabi Z, Sinn E, Chen J, et al. Appended 1,2-naphthoquinones anticancer agents 1: synthesis, structural, spectral and antitumor activities of ortho-naphthaquinone thiosemicarbazone and its transition metal complexes. Inorgan Chim Acta 2004; 57: 271–278
- Kovala-Demertzi D, Miller JR, Kourkoumelis N, Hadjikakou SK, Demertzis MA. Palladium(II) and platinum(II) complexes of pyridine-2-carbaldehyde thiosemicarbazone with potential biological activity. Synthesis, structure and spectral properties. Extended network via hydrogen bond linkages of [Pd(PyTsc)Cl]. Polyhedron 1999; 18: 1005–1013
- Singh NN, Singh SB. Synthesis, characterization and biological properties of manganese(II), cobalt(II), nickel(II), copper(II), zinc(II), chromium(III) and iron(III) complexes with a new thiosemicarbazide derivative. Ind J Chem 2001; 40: 1070–1075
- Agarwal RK, Singh L, Sharma DK. Synthesis, spectral, and biological properties of copper(II) complexes of thiosemicarbazones of Schiff bases derived from 4-aminoantipyrine and aromatic aldehydes. Bioinorgan Chem Appl 2006, Article ID 59509,10 pages
- Garc'ia-Tojal J, Lezama L, Pizarro JL, Insausti M, Arriortua MI, Rojo T. Spectroscopic and magnetic properties of copper(II) complexes derived from pyridine-2-carbaldehydethiosemicarbazone.Structures of [Cu(NO3)-(C7H8N4S)(H2O)](NO3) and [{Cu(NCS)(C7H7N4S)}2]. Polyhedron 1999; 18: 3703–3711
- Labisbal E, Haslow KD, Sousa-Pedrares A, Valde's-Martinez J, Hern'andez-Ortega S, West DX. Copper (II) and nickel (II) complexes of 5-methyl-2-hydroxyacetophenone N(4)-substituted thiosemicarbazones. Polyhedron 2003; 22: 2831–2837
- El-Shazly RM, Al-Hazmi GAA, Ghazy SE, El-Shahawi MS, El-Asmy AA. Spectroscopic, thermal and electrochemical studies on some nickel(II) thiosemicarbazone complexes. Spectrochim Acta—Part A: Mol Biomol Spect 2005; 61: 243–252
- Mostafa SI, El-Asmy AA, El-Shahawi MS. Ruthenium (II) 2-hydroxybenzo-phenone N(4)-substituted thiosemicarbazone complexes. Trans Metal Chem 2000; 25: 470–473
- Chattopadhyay SK, Ghosh SA. A study of Ru(II)complexes of some selected N–S donors. Inorgan Chim Acta 1987; 131: 15–20
- Mukkanti K, Singh RP. Complexes of platinum, rhodium, iridium and ruthenium with a thiosemicarbazone derived from thiophene-2-carboxaldehyde. Trans Metal Chem 1987; 12: 299–301
- Sinha PK, Chakravarty J, Bhattacharya S. Synthesis, characterization, redox properties and reactivities of a group of phenolato complexes of ruthenium(III). Polyhedron 1997; 16: 81–87
- Chakravarty J, Bhattacharya S. Synthesis, characterization, electron-transfer properties and reactivities of a group of ruthenium(II) complexes with RuN2P2X2(X = Cl, Br) coordination spheres. Polyhedron 1994; 13: 2671–2678
- Chattopadhyay SK, Hossain M, Ghosh S, Guha AK. Ligational behaviour of two biologically active N-S donors towards cobalt(III), iron(III), iron(II) and rhodium(III). Trans Metal Chem 1990; 15: 473–477
- Jayabalakrishnan C, Karvembu R, Natarajan K. Synthesis, characterisation, catalytic, and biocidal studies of ruthenium(III) complexes with thiosemicarbazones of.diketoesters. Synth React Inorgan Metal-Organ Nano-Metal Chem 2002; 32: 1099–1113
- Chohan ZH, Pervez H, Khan KM, Supuran CT. Organometallic-based antibacterial and antifungal compounds: Transition metal complexes of 1,1′-diacetylferrocene-derived thiocarbohydrazone, carbohydrazone, thiosemicarbazone and semicarbazone. J Enz Inhibit Med Chem 2005; 20: 81–89
- Chohan ZH, Pervez H, Rauf A, Khan KM, Supuran CT. Isatin-derived antibacterial and antifungal compounds and their transition metal complexes. J Enz Inhibit Med Chem 2004; 19: 417–423
- Singh K, Singh DP, Barwa MS, Tyagi P, Mirza Y. Some bivalent metal complexes of Schiff bases containing N and S donor atoms. J Enz Inhibit Med Chem 2006; 21: 749–755
- Chohan ZH, Shaikh AU, Naseer MM, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic properties of metal-based furanyl derived sulfonamides. J Enz Inhibit Med Chem 2006; 21: 771–781
- Salah S. Uses of o-hydroxybenzoylacetone in the synthesis of some substituted 2-methylchromones chelating agents, and related materials. Ind Eng Chem Res 2001; 40: 37–39
- Saha GC, Khayax K, Islam Md R, Chowdhury Md SK. Synthesis of thiocarbohydrazide, some thiocarbohydroxyhydrazzones and their cyclized products as probes for pharmacological studies. Ind J Chem 1992; 31: 547–550
- Collins CH, Lyne PM. Microbial methods. University Park Press, Baltimore, MD, USA 1970
- Geary WJ. The use of conductivity measurements in organic solvents for the characterization of coordination compound. Coord chem Rev 1971; 7: 81
- Silverstein RM, Bassler GC, Morrill TC. Spectrometric identification of organic compounds. John Wiley & Sons, New York 1991
- Nakamoto K. Infrared spectra of inorganic and coordination compounds. John Wiley & Sons, New York, NY 1970
- Manolov L, Raleva S, Genova P, Savov A, Froloshka L, Dundarova D, Radka Argirova R. Antihuman Immunodeficiency Virus Type 1 (HIV-1) Activity of Rare Earth Metal Complexes of 4-Hydroxycoumarins in Cell Culture. Bioinorg Chem Appl 2006; 2006: 71938
- Dyer JR. Applications of absorption spectroscope of organic compounds. Prentice-Hall, London 1965
- Ueno K, Martel AE. Ultraviolet and visible absorption spectra of metal.chelates of bis-acetylacetoneethylenediimine and related compounds. J Phys Chem 1957; 61: 257–261
- Bailer JC, Emeleus HJ, Nyholm R, Trotman-Dickinson AF. Comprehensive inorganic chemistry. Pergamon Press, Oxford 1975; vol. 3: 517, 1153, 1088, 1048
- Figgs BN. Introduction to ligand field. Wiley, New York 1966, Lever, ABP, Inorganic electronic spectroscopy, 2nd ed., Elsevier, Amsterdam, (1984)
- Baldwin ME. Sulphitobis(ethylenediamine)cobalt(III) complexes. J Chem Soc 1961, 961: 3123–3128
- Williams DH, Fleming I. Spectroscopic methods in organic chemistry. McGraw-Hill, New York 1966
- Levy GC, Nelson G. Carbon-13 nuclear magnetic resonance for organic chemists. John Wiley & Sons, Inc., New York 1972
- Sadler PW. Hydrogen bonding in some thiosemicarbazones and thio amides. J Chem Soc 1961; 957–960
- Sarma BD, Bailer JC, Jr. The stereochemistry of metal chelates with polydentateligands. Part I. J Amer Chem Soc 1955; 77: 5476–5480
- West DX, Padhye SB, Sonawane PB, Chikte RC. Copper (II) complexes of tridentate (ONS) thiosemicarbazones. Asian J Chem Rev 1990; 1: 125–137
- Singh B, Mishra H. Complexes of cobalt(II), nickel(II), copper(II), zinc(II) cadmium(II) and dioxouranium(VI) with thiophene-2-aldehydethiosemicarbazones. J Ind Chem Soc 1986; 63: 692–694
- Thomas J, Parameswaran G. Structural, thermoanalytical and antitumour studies of metal chelates of anthracene-9 carboxaldehyde thiosemicarbazone. Asian J Chem 2002; 14: 1354–1364
- Murthy N, Dharmarajan TS. Synthesis, characterization and biological activity of copper(II) complexes with phenylglyoxal bis-(thiosemicarbazones). Asian J Chem 2002; 14: 1325–1330
- Taylor MR, Glusker JP, Grabe EJ, Minkin JA. The crystal structure of the antitumor agent 3-ethoxy-2-oxobutyraldehyde bis(thiosemicarbazonato) copper(II). Bioinorgan Chem 1974; 3: 189–205
- Bellaci FM, Gasparri FG, Leporati E, et al. Synthesis, characterisation and biological activity of three copper(II) complexes with a modified nitrogenous base: 5- formyluracil thiosemicarbazone. J Inorgan Biochem 1998; 70: 145–154
- William H, Stephen V. Theory and application of microbiological assay. Academic Press, San Diego 1989