Abstract
In recent years, great attention has been given to the search for natural compounds or extracts with the purpose of medical use. Evolvulus alsinoides L. (Convolvulaceae) is a plant used in traditional medicine of East Asia in many indications and has known nootropic and anti-inflammatory activity. However, the bioactive constituents have been described poorly in the literature. Four substances isolated from the ethanol extract of E. alsinoides by means of polyamide and Silica-gel chromatography are reported here. Their molecular structures were determined using NMR analyses. There were identified as scopoletin, umbelliferone, scopolin and 2-methyl-1,2,3,4-butanetetrol. The quantity of these substances was determined using HPLC-UV and GC-FID detection. Antioxidant activity of the isolated substances was measured by DPPH assay using the SIA method. Antioxidant activity and total phenolic content of the prepared fractions are also described. The prepared fractions and isolated substances did not exhibit any significant activity in DPPH test.
Introduction
There are a lot of scientific attention on plants and their secondary metabolites acting as potential drugs against various diseases like cardiovascular disease, neurodegenerative disorders, cancer etc. Citation1-6. In recent, the great interest is given especially in the phytochemical research of traditionally used medical herbs (e.g. Korean, Thailan or Algerian traditionally medicine) [Citation7,Citation8,Citation9].
Evolvulus alsinoides L. belonging to the family Convolvulaceae is a weed from tropical and subtropical swampy regions of the world, mainly of East Asia. It is a prostrate perennial herb with a small woody branched rootstock. Branches are annual, numerous, more than 30 cm long, often prostrate with long hairs. Leaves are small, elliptic, acute, densely hairy and sessile. Flowers are blue, solitary [Citation10,Citation11]. The whole plant is used in Ayurveda as a brain tonic in the treatment of neurodegenerative diseases, asthma and amnesia, and further for antispasmodic, antihaemorrhagic, antioxidant and anti-inflammatory effects Citation12-14. This plant is contained in several preparations with nootropic activity (e. g. Mentat®, Anxocare®) [Citation15,Citation16]. One study showed that E. alsinoides reduced the stress induced in rats [Citation17]. The leaves are also put into cigarettes and smoked in chronic bronchitis and asthma. E. alsinoides has also anti-dysenteric and antiseptic properties [Citation10]. The antioxidant and nootropic preparation Evocen® (consisted of Centella asiatica and Evolvulus alsinoides extracts) was prepared by Avicenna Company Czech Republic recently.
As it was many times before discussed, antioxidants can positive influence the nootropic activity of natural compounds, which are also used as treatment or supplementary treatment of several neurodegenerative disorders, such as Alzheimer's disease or aging [Citation2,Citation4,Citation18].
In this article, we focused our attention on the biological active compounds – constituents presented in E. alsinoides, because there is a lack of such information. Several papers aimed to this topic confirmed the presence of betaine, tannins, carbohydrates, proteins, amino acids, volatile oil, mineral substances (KCl), pentatriacontane, triacontane, β-sitosterol, glycoflavone, 4-methoxyvitexin and phenolic acids (p-hydroxybenzoic, vanillic, protocatechuic and gentisic acid) in E. alsinoides. Alkaloid evolvine was also identified from the whole plant but its chemical structure is till unknown [Citation10,Citation11].
The aim of this study was the phytochemical and antioxidant evaluation of E. alsinoides. That means the preparation of phenolic fractions and determination of their antioxidant activity by DPPH test, and the isolation and identification of bioactive substances from E. alsinoides. Finally, evaluation of antioxidant activity and determination of quantitative content of isolated compounds was performed.
Material and methods
Instruments and materials
NMR spectra were recorded on a Varian Mercury – Vx BB 300 spectrometer: 1H-NMR 300 MHz and 13C-NMR 75.46 MHz. IR spectra were recorded on the spectrophotometer Nicolet Impact 400 in KBr disks, detector DTGS. UV spectra and TPC were determined on a Shimadzu UV-1601 spectrophotometer. Silica gel 60GF254 aluminium plates (Merck) were used for TLC analysis and Camag TCL scanner 3 was used to evaluation of Si-gel plates. Quantitative analyses of (1 – 3) was done on HPLC system Merck, WellChrom pump K-1800, detector DAD K-2700, analytical column RP18 Purospher (Merck) endcapped, 5 μm, (250x 4 mm), pre-column Tessek, SGX C18, CGC 30 × 3 mm, 7 μm, flow-rate 0.3 ml/min, using mixture of 30% ACN/70% water, detection 254 nm. Quantitative analysis of (4) was done on GC Fissons 8000, FID detection. Antioxidant activity was measured on FIAlab 3000 analyser (FIAlab Instruments Inc., Bellevue, WA, USA), 2.5 ml syringe pump, six-ports selector valve, USB 2000-UV/VIS spectrophotometer with LS-1 (Ocean Optics, USA), SMA-Z flow cell (1-cm path length) and FIAlab for Windows version 5.9.126.
Plant material
Branches with leaves of E. alsinoides were of Indian origin and the material was supplied by Avicenna Company, Prague, Czech Republic. The plant material was supplied with the document of authenticity.
Extraction and isolation
The dried powdered plant of E. alsinoides (8.8 kg) was percolated with 95% EtOH (1:15, temp. 22°C) and evaporated in vacuum, yield 393 g. Crude extract was dissolved in 80% MeOH and partitioned with Pe (30–60°C) (4 × 1400 ml, yield 92 g). MeOH layer was evaporated dissolved in H2O (6.9 l) and partitioned with Et2O (5x 1300 ml, yield 70 g). The water residue – polar fraction, yield 230 g. Polar fraction was subjected to polyamide (100–200 mesh) CC using H2O and EtOH 95% to give two fractions; fraction of non-phenolic compounds (FNC) was eluted with H2O (yield 196.5 g), fraction of phenolic compounds (FFC) was eluted with EtOH, yield 12.5 g.
FFC was subjected to Si-gel (100–200 mesh, 1:50) CC using CHCl3/EtOH mixture. A total of 35 sub-fractions were collected on the basis of TLC. Fraction 5 (60 mg) resp. 6 were recrystallized in MeOH and purified to yield (1) (scopoletin, 25 mg) resp. (2) (umbelliferone, 33 mg).
FNC was subjected to Si-gel (40–160 mesh, 1:100) CC using CHCl3/EtOH mixture. A total of 22 final sub-fractions were collected on the basis of TLC. Fraction 15 (55 mg) was recrystallized in MeOH and purified to yield (3) (scopolin, 28 mg). Fraction 18 (650 mg) was recrystallized in the mixture of acetone/propanol (1:1) and purified to yield (4) (2-methyl-1,2,3,4-butanetetrol, 455 mg).
Scopoletin (1). White powder; Mp: 199 – 204 °C, UV (MeOH): max 344 (log ϵ 4.3), 297 (3.9), 253 (3.9), 229 (4.3), 210 (4.3) nm, min 307 (3.8), 271 (3.5), 247 (3.9), 217 (4.2) nm; 1H-NMR (300 MHz, CD3OD): δ 3.89 (3H, s, CH3O), 6.19 (1H, d, J 9.5 Hz, H-3), 6.75 (1H, s, H-8), 7.09 (1H, s, H-5), 7.83 (1H, d, J 9.5 Hz, H-4); 13C-NMR (75 MHz, CD3OD): δ 56.8 (CH3O), 103.9 (C-8), 109.9 (C-5), 112.5 (C-4a), 112.6 (C-3), 146.1 (C-4), 147,1 (C-6), 151.4 (C-8a), 152.9 (C-7), 164.0 (C-2).
Umbelliferone (2). White powder; Mp: 226 – 229°C, UV (MeOH): max 325 (log ϵ 4.3), 253 (3.3), 216 (4.0), 205 (4.2) nm, min 262 (3.0), 251 (3.3), 214 (4.0) nm; 1H-NMR (300 MHz, DMSO-d6): δ 6.18 (1H, d, J 9.5 Hz, H-3), 6.69 (1H, d, J 2.2 Hz, H-8), 6.76 (1H, dd, J1 8.5 Hz, J2 2.2 Hz, H-6), 7.49 (1H, d, J 8.5 Hz, H-5), 7.90 (1H, d, J 9.5 Hz, H-4), 10.56 (1H, bs, OH); 13C-NMR (75 MHz, DMSO-d6): δ 102.4 (C-8), 111.50 (C-3) 111.6 (C-10), 113.3 (C-6), 129.9 (C-5), 144.7 (C-4), 155.7 (C-9), 160.7 (C-2), 161.5 (C-7).
Scopolin (3). White, amorphous powder; Mp: 222 – 225°C, UV (MeOH): max 332 (log ϵ 4.0), 291 (3.9), 227 (4.1), 208 (4.3) nm, min 303 (2.5), 265 (3.5), 216 (4.1) nm; 1H-NMR (300 MHz, DMSO-d6) δ 3.16–3.72 (m, glc), 3.80 (3H, s, OCH3), 5.36 (1H, d, J 7.3 Hz, H-1 glc), 6.32 (1H, d, J 9.5, H-3), 7.15 (1H, s, H-8), 7.28 (1H, s, H-5) 7.95 (1H, d, J 9.6 Hz, H-4); 13C-NMR (75 MHz, DMSO-d6): δ 52.2 (OCH3), 60.8 (C-6′), 69.8 (C-4′), 73.3 (C-2′), 77.0 (C-3′), 77.3 (C5′), 99.8 (C-1′), 103.2 (C-8), 109.8 (C-5), 112.5 (C-10), 113.5 (C-3), 144.4 (C-4), 146.2 (C-6), 149.1 (C-9), 150.1 (C-7), 160.8 (C-2).
2-methyl-1,2,3,4-butanetetrol (4). Transparent crystals; Mp: 81 – 84°C, Optical rotation:
+19.6°(c 3.5 in H2O); 1H-NMR (300 MHz, CD3OD): δ 1.10 (3H, s, CH3), 3.43 (1H, d, J 11.3 Hz, H1), 3.52 (1H, d, J 11.3 Hz, H1), 3.64–3.53 (2H, m, H-3, H-4), 3.79 (1H, dd, J 9.6 Hz, J 1.9 Hz, H-4); 13C-NMR (75 MHz, CD3OD): δ 19.7 (C-1), 63.8 (C-4), 68.4 (C-1), 74.9 (C-2), 76.1 (C-3).
Quantitative evaluation of isolated substances
EtOH extract of E. alsinoides was prepared to treat HPLC of (1–3). 250.0 mg of dried plant was extracted with EtOH 60% (w/w, 3 × 10.0 mL). Ultrasonic bath Sonorex was used for extraction (time 30 min., temp. 50°C, sonication level 10). Collected extracts were evaporated in vacuum. Three samples were prepared for HPLC according to this method; every sample was dissolved in 1.0 mL MeOH and send to HPLC system [Citation19].
H2O extract of E. alsinoides was prepared to treated GC-FID analysis of (4). 250.0 mg of dried plant was extracted with 50.0 mL of H2O (H2O bath, temp. 50°C, time 2 h), then maceration over night and filtration. After filtration was the plant further extracted with 2 × 15.0 mL of H2O by using ultrasonic bath Sonorex (time 20 min., temp. 50°C, sonication level 10). Collected extracts were evaporated. Three samples were prepared. Every sample was dissolved in 10.0 ml 90% MeOH. 100.0 μL of each sample was derivatized with trisilil TBT (Pierce), time 1 h, 70°C and sent to GC-FID system [Citation20].
DPPH free radical scavenging activity
PC-controlled sequential injection analysis (SIA) system equipped with spectrophotometric DAD was used for evaluation of radical scavenging activity of extracts, natural and synthetic substances Citation21-23. The decrease of the absorbance of DPPH (2,2′diphenyl-1-pikrylhydrazyl) measured at 525.0 nm is related to concentration of antioxidants in the test samples. Ascorbic acid and trolox was used as standards. Antioxidant activity of plant extracts, FFC and four isolated substances (1–4) was measured. The samples and standards were dissolved in 50% EtOH in concentration 1, 0.5, 0.25, 0.1, 0.05, 0.025, 0.01 mg/ml and measured.
Total phenolic content
Total phenolic content (TPC) in measured fractions was determined using the Folin–Ciocalteau method [Citation24]. TPC is expressed as percentage of gallic acid.
Results and discussion
From the prepared plant extracts, the fraction of phenolic compounds (FFC) (EC50 0.314 mg/mL), water residue (EC50 0.494 mg/mL) and crude ethanol extract (EC50 0.548 mg/mL) were the most active against DPPH radical. Compared to these fractions fractions, DPPH radical scavenging activity of petrolether extract, diethylether extract and fraction of non-phenolic compounds (FNC) was low (EC50 values > 1 mg/mL).
Two compounds (1,2) from FFC and two compounds (3,4) from FNC were isolated from E. alsinoides by using chromatographic methods. Their structures were determined by NMR analysis, IR spectra and physicochemical data, which were in accordance with literature data Citation25-27.
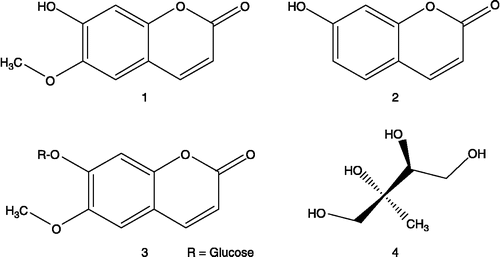
The structures of isolated compounds are shown in .
Three isolated compounds have a simple coumarin structures: scopoletin (1), umbelliferone (2), scopolin (3). Generally, coumarins comprise a large class of phenolic compounds occurring in plants. They possess anti-inflammatory and antioxidant activities and have been used to treat various ailments such as cancer, burns, cardiovascular and rheumatoic diseases [Citation28,Citation29]. Scopolin is the glycoside of scopoletin and D-glucose. The last isolated substance has the structure of polyol - 2-methyl-1,2,3,4-butanetetrol (4). According to the present knowledge, none of isolated compounds were considered as constituent of E. alsinoides.
Scopoletin and umbelliferone show intensive blue fluorescent spot in UV 365 nm (Rf 0.56 and Rf 0.47 in CHCl3:MeOH - 95:5) and 2-methyl-1,2,3,4-butanetetrol shows intensive violet spot with pink border in VIS after spraying with vanillin-sulphuric acid reagent and after heating of the chromatogram at 110°C for 5 minutes (Rf 0.41 in CHCl3:MeOH - 7:3). Accordingly, we could recommend the scopoletin (1), umbelliferone (2) and 2-methyl-1,2,3,4-butanetetrol (4) as markers of qualitative and quantitative analysis of commercial preparations with E. alsinoides.
The results of antioxidant activity of isolated compounds and antioxidant standards (acid ascorbic, trolox) are shown in . These results demonstrate that the isolated coumarins 1 – 3 did not exhibit antioxidant activity in the range of measured concentrations. The low antioxidant activity of scopoletin is in a good agreement with data obtained by the study of Shaw et al. [Citation30].
Table I. EC50 values of DPPH free radical scavenging activity of standards and compounds 1 – 4.
According to the results obtained, DPPH radical scavenging activity of polyol (4) was low, too. This result was not surprising because of any existing scientific information about significant antioxidant activity of this type of compound.
The results of antioxidant activity and total phenolic content of phenolic sub-fractions (FFC) are shown in . From the total number of 35 prepared phenolic fractions, only fractions no. 12 – 35 were analyzed. The fractions no. 1 – 11 were of insufficient yields for analysis conduction. The most antioxidant potent were fractions no. 31 or 28. The EC50 values of these fractions were 0.230 mg/mL and 0.258 mg/mL. These EC50 values are approximately 33 times higher than the activity of trolox (EC50 0.0072 mg/mL) respectively 29 times higher than the activity of ascorbic acid (EC50 0.0077 mg/mL). These results indicate low antioxidant power of all tested fractions. Though, the results of total phenolic content in FFC (no. 12 – 35) are generally high (the lowest value is 11.721% for fraction no. 34), no significant correlation between the antioxidant power and the content of phenolic compounds was found.
Table II. Total phenolic content and EC50 value of DPPH free radical scavenging activity of 24 phenolic fractions from E. alsinoides. (TPC = Total Phenolic Content =% of gallic acid equivalents in the dried fraction; DPPH =2,2′-diphenyl-1-picrylhydrazyl (EC50 mg/mL)).
The results of quantitative analysis of isolated substances are shown in . The content of coumarins 1 – 3 (0.009% – 0.027%) is low in comparison with the content of 2-methyl-1,2,3,4-butanetetrol (4) (0.87%). The most included 2-methyl-1,2,3,4-butanetetrol (4) is known putative precursor of isoprenoids in mevalonate-independent pathway, nevertheless is not considered as bioactive plant secondary metabolite [Citation31].
Table III. Results of quantitative analyses. Compounds no. 1 – 4.
In conclusion, E. alsinoides is a widely used plant in traditional medicine of East Asia in many indications Citation12-14. This plant is also contained in several commercial preparations with antioxidant and nootropic activity [Citation15,Citation16]. In this study we performed a phytochemical research of E. alsinoides with the purpose to isolate and identify antioxidant active substances. During the isolation procedure, we prepared a fraction of phenolic compounds (FFC), which are generally known as bioactive plant substances [Citation2,Citation32]. FFC was examined for antioxidant and phenolic substances in detail, however only two weak antioxidant active coumarins (1,2) were isolated. Coumarin (3) and polyol (4) were isolated from the fraction of non-phenolic compounds (FNC). Obtained results of antioxidant activities of E. alsinoides from DPPH assays were not as high as we expected. For the expansion of our results, more antioxidant tests with different action mechanisms and also in-vivo studies with E. alsinoides are required.
Acknowledgements
This project was supported by the Research Projects 169/2004/B-BIO/FAF and 124/2005/B-BIO/FaF of Charles University Grant Agency and Research Project MSM0021620822.
References
- Balasundram N, Sundram K, Samman S. Food Chem 2006; 99: 191–203
- Kondratyuk TP, Pezzuto JM. Pharm Biol 2004; 42: 46–63
- Koleckar V, Opletal L, Brojerova E, Rehakova Z, Cervenka F, Kubikova K, Kuca K, Jun D, Polasek M, Kunes J, Jahodar L. J Enz Inhib Med Chem 2007, in press
- Cantuti-Castelvetri I, Shukitt-Hale B, Joseph JA. Int J Dev Neurosci 2000; 18: 367–381
- Yang L, Gong J, Wang F, Zhang Y, Wang Y, Hao X, Wu X, Bai H, Stockigt J, Zhao Y. J Enz Inhib Med Chem 2006; 21: 399–404
- Koleckar V, Brojerova E, Opletal L, Jun D, Kuca K. Ces Slov Farm 2007; 56: 73–76
- Chang WC, Sei CK, Soon SH, Bong KC, Hye JA, Min YL, Sang HP, Soo KK. Plant Sci 2002; 163: 1161–1168
- Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N. Food Chem 2005; 92: 491–497
- Djeridane A, Yousfi M, Nadjemi B, Maamri S, Djireb F, Stocker P. J Enz Inhib Med Chem 2006; 21: 719–726
- Krishnakumar SPR, et al. Selected medicinal plants of India: Monograph of identity. Safety and clinical usage. Chemexcil, Bombay 1992; 151–154
- Daniel M. Medical plants: Chemistry and properties. Science publishers, Enfield 2006; 21
- Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, Tripathi PC, Seal T, Mukherjee B. J Ethnopharmacol 2003; 84: 131–138
- Ganju L, Karan D, Chanda S, Srivastava KK, Sawhney RC, Selvamurthy W. Biomed Pharmacother 2003; 57: 296–300
- Achliya Girish S, Barabde U, Wadodkar S, Dorle A. Indian J Physiol Pharmacol 2004; 36: 159–162
- Kulkarni SK, Verna A. Indian J Physiol Pharmacol 1992; 36: 29
- Kumar A, Kulkarni SK. Phytother Res 2006; 20: 538–541
- Siripurapu KB, Gupta P, Bhatia G, Maurya R, Nath C, Palit G. Pharmacol Biochem Behav 2005; 81: 424–432
- Werneke U, Turner T, Priebe S. Br J Psychiatry 2006; 188: 109–121
- Hawryl MA, Soczewinski E, Dzido TH. J Chromatogr 2000; 886: 75–81
- Wang W, Gyorgy V, Dommisse R, Loones K, Claeys M. Rapid Commun Mass Spectrom 2004; 18: 1787–1797
- Polasek M, Skala P, Opletal L, Jahodar L. Anal Bioanal Chem 2004; 379: 754–758
- Rehakova Z, Koleckar V, Cervenka F, Jahodar L, Saso L, Opletal L, Jun D, Kuca K. Toxicol Mech Method 2007, in press
- Koleckar V, Jun D, Opletal L, Jahodar L, Kuca K. J Appl Biomed 2007; 5: 81–84
- Pharmacopoeia Bohemoslovaca. Editio quarta. Avicenum, Prague 1987; 105, volume 1
- Zolek T, Paradowska K, Wawer I. Solid State Nucl Magn Reson 2003; 23: 77–87
- Lee JH, Ku CH, Baek NI, Kim SH, Park HW, Kim DK. Arch Pharm Res 2004; 27: 40–43
- Kis K, Wungsintaweekul J, Eisenreich W, Zenk MH, Bacher A. J Org Chem 2000; 65: 587–592
- Kontogiorgis CA, Savvoglou K, Hadjipavlou-Litina DJ. J Enz Inhib Med Chem 2006; 21: 21–29
- Kontogiorgis C, Hadjipavlou-Litina D. J Enz Inhib Med Chem 2003; 18: 63–69
- Shaw CY, Chen CH, Hsu CC, Chen CC, Tsai YC. Phytother Res 2003; 17: 823–825
- Zhong JJ, Yue CJ. Adv Biochem Eng Biotechnol 2005; 100: 53–88
- Bravo L. Nutr Rev 1998; 56: 317–333