Abstract
Phytochemical investigations on the alkaloidal fraction of the whole plant of the Isatis tinctoria led to the isolation of the alkaloids 1-6., 3′-Hydroxyepiglucoisatisin (3), Epiglucoisatisin (2) were found to be potent urease inhibitors in a concentration-dependent manner with IC50 values 25.63 ± 0.74, 37.01 ± 0.41 and 31.72 ± 0.93, 47.33 ± 0.31 μM against Bacillus pasteurii & Jack bean urease, respectively. Compounds 3 and 2 also showed potent inhibitory potential against α-chymotrypsin with IC50 values of 23.40 ± 0.21 and 27.45 ± 0.23 μM, respectively.
Introduction
The genus Isatis, belonging to the family Brassicaseae, comprises 50 species mainly distributed in Irano-Turanian region. In Pakistan it is represented by seven species [Citation1]. Isatis tinctoria or woad is a common plant cultivated throughout the centuries to produce the blue dye indigo. Nowadays, woad is also used in Chinese folk and modern medicine [Citation2]. “Ban-Lan-Gen” is one of the most commonly used traditional Chinese medicines for antipyretic, anti-inflammatory, antiviral and detoxifying purposes. Its original source was considered to be the dried roots of three plants, Isatis indigotica, Isatis tinctoria and Strobilanthes cusia [Citation3,4]. In a recent nationwide investigation, the roots of Isatis indigotica have been identified as the main source of “Ban-Lan-Gen” and recorded in Chinese Pharmacopoeia (1990 edn) [Citation5]. The ethano pharmacological importance of the genus Isatis prompted us to investigate the chemical constituents of Isatis tinctoria, which is an annual or biennial herb, found in northern part of Pakistan and is used by local physicians for the treatment of viral diseases. Our previous work on Isatis costata has resulted oxindole alkaloids [Citation6,7].
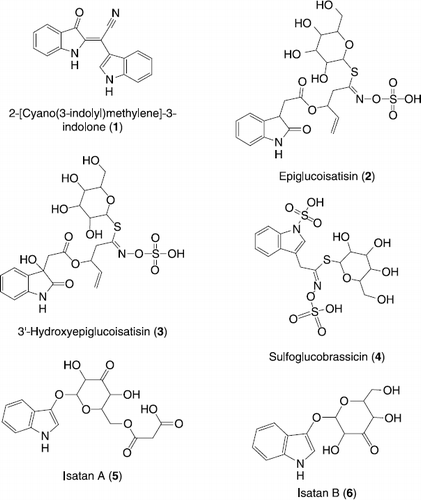
An ethanolic extract of this plant showed strong toxicity in brine shrimp lethality test. Further pharmacological screening revealed strong inhibitory activity in the alkaloidal fraction against the enzymes urease and α-chymotrypsin, which prompted us to conduct phytochemical studies on this plant. As a result six alkaloids, 2-[Cyano(3-indolyl)methylene]-3-indolone (1) [Citation8], Epiglucoisatisin (2) [Citation9], 3′-Hydroxyepiglucoisatisin (3) [Citation9], Sulfoglucobrassicin (4) [Citation10], Isatan A (5) [Citation11] and Isatan B (6) [Citation11] were isolated ().
Ureases (E.C 3.5.1.5) have been shown to be an important virulence determinant in the pathogenesis of many clinical conditions, which is detrimental for human and animal health as well as for agriculture. Urease is directly involved in the formation of infection stones and contributes to the pathogenesis of urolithiasis, pyelonephritis, ammonia and hepatic encephalopathy, hepatic coma, urinary catheter encrustation [Citation12,13]. It is also known to be a major cause of pathologies induced by Helicobacter pylori (HP), which allows bacteria to survive at low pH of the stomach during colonization and, therefore, plays an important role in the pathogenesis of gastric and peptic ulcer (including cancer) [Citation13]. In agriculture, high urease activity causes significant environmental and economic problems by releasing abnormally large amounts of ammonia into the atmosphere during urea fertilization. This further induces plant damage primarily by depriving them from their essential nutrient and secondly ammonia toxicity, which increase the pH of the soil [Citation14,Citation15]. Therefore strategies based on urease inhibition are now considered as the first line of treatment for infections caused by urease producing bacteria.
The physiological roles of serine protease inhibitors have been clearly established. It has been proposed that they are part of plants natural defense system against insect predation and function by inhibiting insect proteinases [Citation16–18]. Hence these inhibitors have gained attention as possible sources of engineered resistance against pests and pathogens for transgenic plants expressing heterologous inhibitors [Citation18] [Citation9]. Tobacco plants transformed with gene coding serine protease inhibitor has been shown to posses’ insect pest resistance [Citation19]. Bowman-Birk inhibitors have also been shown to be efficient tumor suppressor agents in vivo and in vitro [Citation20]. Serine proteases such as chymotrypsin and trypsin are involved in the destruction of certain fibrous proteins [Citation21]. Chronic infection by hepatitis C virus can lead to the progressive liver injury, cirrhosis, and liver cancer. A chymotrypsin like serine proteases known as NS3 protease, which has very similar active site as chymotrypsin is taught to be essential for viral replication and has become target far anti-HIV drugs [Citation22]. So search for new effective inhibitors of serine proteases is an urgent need for the drug development.
In the current study we have described the urease and protease inhibitory activities of the alkaloids (1-6) which were isolated from Isatis tinctoria and although the structures of the compounds were published previously not their urease/protease activities.
Materials and methods
Plant material
The whole plant material was collected in April 2006 from N. W. F. P Swat and identified as Isatis tinctoria by Nisar Ahmad, Centre of Biotechnology, University of Peshawar, Pakistan. A voucher specimen (BPU-279) is deposited in the herbarium of the Department of Botany, University of Peshawar, Pakistan.
Extraction and isolation
The shade-dried whole plants (10 kg) were chopped and extracted thrice with EtOH (60 L) at rt for 96 h. The ethanolic extract was evaporated in vacuo to give a dark greenish residue (210 g), which was partitioned between EtOAc and water. The water fraction was basified with 10% NH4OH and extracted with CH2Cl2. The CH2Cl2 fraction (25 g) was subjected to column chromatography eluting with n-hexane-EtOAc in increasing order of polarity to obtain three sub fractions. The sub fractions on repeated column chromatography afforded compounds 1-6.
In vitro Urease inhibition assay
Reaction mixtures comprising 25 μL of enzyme (Jack bean and Bacillus pasteurii Urease) solution incubated for 30 min with 5 μL test compounds at 30°C for 15 min in 96-well plates and then 55 μL of buffers containing 100 mM urea were incubated for 15 min. At the end final urease activity was determined by measuring ammonia production using the indophenol method as described by Weatherburn [Citation15]. Briefly, 45 μL each of phenol reagent (1% w/v phenol and 0.005% w/v sodium nitroprusside) and 70 μL of alkali reagent (0.5% w/v NaOH and 0.1% active chloride NaOCl) were added to each well. The increasing absorbance at 630 nm was measured after 50 min, using a microplate reader (Molecular Device, USA). All reactions were performed in triplicate in a final volume of 200 μL. The results were processed by using SoftMax Pro software (Molecular Device, USA). All the assays were performed at pH 8.2 potassium phosphate buffer (0.01 M K2HPO4.3H2O, 1 mM EDTA, 0.01 M LiCl and HCl were used to adjust pH). Percentage inhibitions were calculated from the formula 100 − (ODtestwell/ODcontrol) × 100). Thiourea was used as the standard inhibitor of urease. We purchased both ureases from Sigma.
In vitro Chymotrypsin inhibition assay
The chymotrypsin inhibitory activity of compounds 1-6 were performed by the method of Cannel et al. [Citation23]. Chymotrypsin (9 units/ mL of 50 mM Tris-HCl buffer pH 7.6; Sigma Chemicals (St. Louis, Mo, USA) was preincubated with various concentrations of test compounds for 20 min at 25°C, respectively, then 100 μL of substrate solution (N-succinyl-phenylalanine-p-nitroanilide, 1 mg/ mL of 50 mM Tris-HCl buffer pH 7.6) was added to start the enzyme reaction. The absorbance of released p-nitroaniline was continuously monitored at 410 nm until a significant color change was achieved. The final DMSO concentration in the reaction mixture was 7%.
Determination of IC50 values
The concentrations of the test compounds that inhibited the hydrolysis of substrates by 50% (IC50) were determined by monitoring the effect of various concentrations of these compounds in the assays on the inhibition values. The IC50 values were then calculated using the EZ-Fit Enzyme Kinetics program (Perrella Scientific Inc., Amherst, USA).
Results and discussion
The ethanolic extract of Isatis tinctoria was partitioned between EtOAc and water. Alkaloids liberated from the aqueous fraction with 10% NH4OH were extracted out with CH2Cl2. Column chromatography of CH2Cl2 fraction provided the alkaloids (1-6).
Urease inhibitory activities of alkaloids 1-6
Compounds 3 and 2 displayed potent inhibitory potential against the enzyme with IC50 values 25.63 ± 0.74, 37.01 ± 0.41 and 31.72 ± 0.93, 47.33 ± 0.31 μM against Bacillus pasteurii & Jack bean urease, respectively. Compounds 1, 4, 5 and 6 showed significant inhibitory activity against both ureases (). The standard inhibitor of Bacillus pasteurii & Jack bean urease (thiourea) had IC50 values of 15.06 ± 0.72 and 21 ± 0.11 μM, respectively.
Table I. In vitro inhibition of Urease and α-chymotrypsin by alkaloids 1-6.
α-chymotrypsin inhibitory activities of alkaloids 1-6
3′-Hydroxyepiglucoisatisin (3) and Epiglucoisatisin (2) inhibited α-chymotrypsin enzyme in a concentration-dependent manner with IC50 values 23.4 and 27.45 μM, respectively, whereas the positive control, chymostatin, had an IC50 value 7.01 μM (SEM is shown for each IC50 value in ). Therefore, both the isolated compounds 3 and 2 have significant potential to bind and inhibit α-chymotrypsin enzyme. On the other hand, 2-[Cyano(3-indolyl)methylene]-3-indolone (1), Sulfoglucobrassicin (4), Isatan A (5) and Isatan B (6) displayed moderate inhibitory potential against α-chymotrypsin ().
From the results () it is clear that the compounds with more hydroxyl/carbonyl moieties are the most active inhibitors of both the enzymes, compound 3 with more hydroxyl groups than other compounds is the most potent with IC50 value of 25.63 ± 0.74 & 31.72 ± 0.93 μM against Bacillus pasteurii & Jack bean urease, respectively and also showed potent inhibitory potential against α-chymotrypsin with an IC50 values of 23.40 ± 0.21. Compound 6 with three glucosidal hydroxyl groups showed the least inhibition in this series. This gradual increase in inhibition with the increasing number of hydroxyl/carbonyl groups may be apparently due to chelation of these moieties with the active site of the enzymes which enhances the inhibitory potential of the compounds. Clearly there are many questions regarding the mode of action of these ligands that need to be answered and these answers of these questions will play an important role in the development of future generations of these inhibitors. For that reason we are synthesizing several derivatives of compounds 1-6 to be evaluated against urease and α-chymotrypsin in vitro through STD NMR and molecular dynamics simulation and kinetics studies to establish a detailed mechanism of inhibition of these compounds.
Conclusion
In conclusion, our search for urease and protease inhibitory constituents from Isatis tinctoria has resulted in the isolation of alkaloids 1-6, as potential agents in the treatment urease- and protease-associated complications. However, a further in vivo study would help in providing further insight into the pharmacological properties of these lead compounds.
References
- YJ Nasir, and SI Ali. (1989). Flora of Pakistan. National Herbarium Pakistan Agriculture Research Council, Islamabad 191:94.
- M Pinkas, W Peng, M Torck, and F Trotin. Plants Medicinal Chinoises Paris: Maloin; (1996). p 86.
- New Medical College. Jiangsu, Dictionary of Chinese crude Drugs Shanghai: Scientific and Technological Press; (1985) 1250–1252.
- Institute of Materia Medica. Chinese Academy of Medical Sciences. In. YZ Zhong. editor, 1 Beijing: Peoples Health Press; (1979).
- Ministry of Public Health. Chinese Pharmacopoeia Beijing: part-I: Peoples Health Press; (1990). p 172.
- I Fatima, I Ahmad, SA Nawaz, A Malik, N Afza, G Lutfullah, and MI Choudhary. (2006). Enzymes inhibition studies of oxindole alkaloids from Isatis costata. Heterocycles 68:1421–1428.
- I Fatima, I Ahmad, I Anis, A Malik, and N Afza. (2007). Isatinones A and B, New antifungal oxindole alkaloids from Isatis costata. Molecules 12:155–162.
- WS Chen, B Li, WD Zhang, GJ Yang, and CZ Qiao. (2001). A new alkaloid from the root of Isatis indigotica Fort. Chin Chem Lett 12:501–502.
- A Frechard, N Fabre, C Pean, S Montaut, MT Fauvel, P Rollin, and I Fouraste. (2001). Novel indole-type glucosinolates from woad (Isatis tinctoria L.). Tetrahedron Lett 42:9015–9017.
- IJ Cox. (1984). NMR spectra (1H, 13C) of glucosinolates. Carbohydr Res 132:323–329.
- C Oberthur, B Schneider, H Graf, and M Hamburger. (2004). The Elusive indigo precursors in woad (Isatis tinctoria L.) - Identification of the major indigo precursor, Isatan A, and a structure revision of Isatan B. Chem Biodiversity 1:174–182.
- HLT Mobley, MD Island, and RP Hausinger. (1995). Molecular-biology of microbial ureases. Microbial Rev 59:451–480.
- HLT Mobley, and RP Hausinger. (1989). Microbial ureases: Significance, regulation and molecular characterisation. Microbial Rev 53:85–108.
- JM Bremner. (1995). Recent research on problems in the use of urea as a nitrogen fertilizer. Fert Res 42:321–329.
- MW Weatherburn. (1967). Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–973.
- D Boulter, AMR Gatehouse, and V Hilder. (1989). Use of cowpea trypsin inhibitor (CpTi) to protect plants against insect predation. Biotechnol Adv 7:489–496.
- CA Ryan. (1990). Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annu Rev Phytopathol 28:425–449.
- SA Masoud, LB Jhonson, FF Whiteand, and GR Reeck. (1993). Expression of a cysteine proteinase inhibitor (oryzacystatin-I) in transgenic tobacco plants. Plant Mol Biol 21:655–663.
- VA Hilder, AMR Gatehouse, SE Sheerman, RF Barker, and D Boulter. (1987). A novel mechanism of insect resistance engineered into tobacco. Nature 330:160–163.
- LA Calderon, RCL Teles, JRSA Leite, CJ Bloch, SA Stolfi-Filho, and SM Freitas. (2001). Serine protease inhibitors from Amazon Leguminosae seeds: Purification and preliminary characterization of two chymotrypsin inhibitors from Inga umbratica. Prot Pep Lett 8:485–493.
- PM Starkey. (1977). The effect of human neutrophil elastase and cathepsin G on the collagen of cartilage, tendon, and cornea. Acta Biol Med Germ 36:1549–1554.
- AK Patick, and KE Potts. (1998). Protease Inhibitors as Antiviral Agents. Clin Microbiol Rev 11:614–627.
- RJP Cannel, SJ Kellam, AM Owsianka, and JM Walker. (1988). Results of a large scale screen of microalgae for the production of protease inhibitors. Planta Med 54:10–14.