Abstract
A new series of 3-(4-ethylphenyl)-2-substituted amino-3H-quinazolin-4-ones were synthesized by reacting the amino group of 2-hydrazino-3-(4-ethylphenyl)-3H-quinazolin-4-one from 4-ethyl aniline with a variety of aldehydes and ketones. The title compounds were investigated for analgesic, anti-inflammatory and ulcerogenic index activities. The compound 2-(N′-3-pentylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS2) emerged as the most active compound of the series and was moderately more potent than the reference standard diclofenac sodium. Interestingly the test compounds showed only mild ulcerogenic potential when compared to aspirin.
Keywords::
Introduction
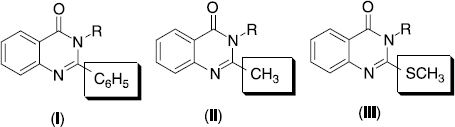
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed for the treatment of acute and chronic inflammation, pain, and fever. Most of NSAIDs act via inhibition of cyclooxygenase, thus preventing prostaglandin biosynthesis. However, this mechanism of action is also responsible for their main undesirable effects, gastrointestinal (GI) ulceration and, less frequently, nephrotoxicity. The increase in hospitalization and deaths due to GI-related disorders parallels the increased use of NSAIDs. Therefore, the discovery of new safer anti-inflammatory drugs represents a challenging goal for such a research area [Citation1–4]. On our going medicinal chemistry research program we found that quinazolines and condensed quinazolines exhibit potent central nervous system (CNS) activities including analgesic, anti-inflammatory [Citation5] and anticonvulsant behavior [Citation6]. Quinazolin-4(3H)-ones with 2,3-substitution are reported to possess significant analgesic, anti-inflammatory [Citation7,8] and anticonvulsant activities [Citation9]. Earlier we have documented some lead 2-phenyl-3-substituted quinazolines [Citation10] (, I), 2-methyl-3-substituted quinazolines [Citation11] (, II), 2-methylthio-3-substituted quinazaolines [Citation12] (, III), and 2,3-disubstituted quinazolines [Citation13] that exhibited good analgesic and anti-inflammatory properties. The present study is an extension of our ongoing efforts towards the development and identification of new molecules for analgesic and anti-inflammatory activities with minimal gastrointestinal ulceration side effects. With this background in the present work we have synthesized a series of 3-(4-ethylphenyl)-2-substitutedamino-3H-quinazolin-4-ones. The synthesized compounds were tested for their analgesic, anti-inflammatory and ulcerogenic index behavior.
Materials and methods
Chemistry
Melting points were taken in open capillaries on a Thomas Hoover melting point apparatus and are uncorrected. Infrared spectra were recorded on an FT-IR Perkin-Elmer spectrometer. The 1H-NMR spectra were recorded on a DPX-300 MHz Bruker FT-NMR spectrometer. The chemical shifts were reported as parts per million (δ ppm) with TMS as internal standard. Mass spectra were obtained on a JEOL-SX-102 instrument using fast atom bombardment (FAB positive). Elemental analysis was performed on a Perkin-Elmer 2400 C,H,N analyzer and values were within the acceptable limits of the calculated values( ± 0.4%). The progress of the reaction was monitored on silica gel plates (Merck) using chloroform-methanol (9:1) as a solvent system. Iodine was used as a developing agent. Spectral data (IR, NMR and mass spectra) confirmed the structures of the synthesized compounds and the purity of these compounds was ascertained by microanalysis. All chemicals and reagents were obtained from Aldrich (USA), Lancaster (UK) or Spectrochem Pvt.Ltd (India) and were used without further purification.
3-(4-Ethylphenyl)-2-thioxo-2,3-dihydro-1H-quinazolin-4-one (4)
A solution of 4-ethyl aniline 1 (0.02 mol) in dimethyl sulfoxide (10 mL) was stirred vigorously. To this was added carbon disulphide (1.6 mL) and aqueous sodium hydroxide 1.2 mL (20 molar solution) drop wise during 30 min with stirring. Dimethyl sulphate (0.02 mol) was added gradually at 5-10°C. The reaction mixture was stirred for 2 h and poured into ice-water. The solid obtained was filtered, washed with water, dried and recrystallized from ethanol. Methyl anthranilate (0.01 mol) and the above prepared N-(4-ethylphenyl)-methyl dithiocarbamic acid (0.01 mol), were dissolved in ethanol (20 mL). To this anhydrous potassium carbonate (100 mg) was added and refluxed for 22 h. The reaction mixture was cooled in ice and the solid separated was filtered and purified by dissolving in 10% alcoholic sodium hydroxide solution and re-precipitated by treating with dilute hydrochloric acid. The solid obtained was filtered, washed with water, dried and re-crystallized from ethanol. Yield = 81%, mp 265-266°C; IR (KBr) cm− 1: 3210 (NH), 1690 (C = O), 1218 (C = S); 1H NMR (CDCl3) δ: 1.3-1.4 (t, 3H, CH2CH3), 2.1-2.2 (q, 3H, CH2CH3), 7.3-8.0 (m, 8H, ArH), 10.1 (br s, 1H, NH, D2O Exchangeable); MS (m/z) 282 (M+). Anal. Calcd for C16H14N2OS: C, 68.06; H, 5.00; N, 9.92. Found: C, 68.09; H, 5.02; N, 9.86%.
3-(4-Ethylphenyl)-2-methylsulfanyl-3H-quinazolin-4-one (5)
The 3-(4-ethylphenyl)-2-thioxo-2,3-dihydro-1H-quinazolin-4-one 4 (0.01 mol) was dissolved in 40 mL of 2% alcoholic sodium hydroxide solution. To this reaction mixture dimethyl sulphate (0.01 mol) was added drop wise with stirring. The stirring was continued for 1 h, the reaction mixture was then poured into ice water. The solid obtained was filtered, washed with water, dried and recrystallized from ethanol-chloroform (75:25) mixture. Yield = 85%, mp 151-153°C; IR (KBr) cm− 1: 1681 (C = O); 1H NMR (CDCl3) δ: 1.5-1.6 (t, 3H, CH2CH3), 2.3-2.4 (q, 3H, CH2CH3), 2.7 (s, 3H, SCH3), 7.2-7.8 (m, 8H ArH); MS (m/z) 296 (M+). Anal. Calcd for C17H16N2OS: C, 68.89; H, 5.44; N, 9.45. Found: C, 68.86; H, 5.47; N, 9.41%.
3-(4-Ethylphenyl)-2-Hydrazino–3H-quinazolin-4-one (6)
The 3-(4-ethylphenyl)-2-methylsulfanyl-3H-quinazolin-4-one 5 (0.01 mol) was dissolved in ethanol (25 mL). To this hydrazine hydrate (99%) (0.1 mol) and anhydrous potassium carbonate (100 mg) was added and refluxed for 39 h. The reaction mixture was cooled and poured into ice water. The solid so obtained was filtered, washed with water, dried and recrystallized from chloroform-benzene (25:75) mixture. Yield = 85%, mp 187-189°C; IR (KBr) cm− 1: 3362, 3281 (NHNH2), 1686 (C = O); 1H NMR (CDCl3): δ 1.6-1.7 (t, 3H, CH2CH3), 2.2-2.3 (q, 3H, CH2CH3) 5.3 (br s, 2H, NH2, D2O Exchangeable), 7.4-8.1 (m, 8H, ArH), 9.8 (br s, 1H, NH, D2O Exchangeable); MS (m/z) 280 (M+). Anal. Calcd for C16H16N4O: C, 68.55; H, 5.75; N, 19.99. Found: C, 68.59; H, 5.74; N, 19.95%.
General synthetic procedure for 3-(4-ethylphenyl)-2-substitutedamino-3H-quinazolin-4-ones (A1-A15)
A mixture of 2-hydrazino-3-(4-ethylphenyl)-3H-quinazolin-4-one (6) (0.004 mol) and appropriate ketones/aldehydes (0.004 mol) in glacial acetic acid was refluxed for 36 h. The reaction mixture was poured into ice water. The solid obtained was recrystallized from ethanol.
2-(N′-2-Butylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS1)
Yield = 71%, mp 265-266°C; IR (KBr) cm− 1: 3268 (NH), 1683 (C = O), 1612 (C = N); 1H-NMR (CDCl3): δ 1.0-1.1 (q, 2H, CH2CH3), 1.3-1.4 (q, 3H, CH2CH3) 1.6-1.7 (t, 3H, CH2CH3), 1.8-1.9 (t, 3H, CH2CH3), 2.3-2.4 (s, 3H, CH3), 7.1-7.7 (m, 8H, ArH), 8.3 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 334 (M+). Anal. Calcd. for C20H22N4O: C, 71.83; H, 6.63; N, 16.75. Found: C, 71.79; H, 6.66; N, 16.73%.
2-(N′-3-Pentylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS2)
Yield = 80%, mp 282-283°C; IR (KBr) cm− 1: 3371 (NH), 1677 (C = O), 1611 (C = N); 1H-NMR (CDCl3): δ 1.0-1.2 (m, 4H, (CH2CH3)2), 1.5-1.7 (m, 6H, (CH2CH3)2), 1.9-2.0 (t, 3H, CH2CH3), 2.4-2.5 (q, 3H, CH2CH3), 7.4-8.0 (m, 8H, ArH), 8.6 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 348 (M+). Anal. Calcd. for C21H24N4O: C, 72.38; H, 6.94; N, 16.08. Found: C, 72.35; H, 6.90; N, 16.06%.
2-(N′-2-Pentylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS3)
Yield = 78% yield, mp 268-269°C; IR (KBr) cm− 1: 3268 (NH), 1685 (C = O), 1617 (C = N); 1H-NMR (CDCl3): δ 1.3-1.4 (t, 2H, CH2CH2CH3), 1.5-1.6 (sext, 2H, CH2CH2CH3), 1.9-2.0 (t, 3H, CH2CH3), 2.2-2.3 (q, 3H, CH2CH3), 2.5-2.6 (t, 3H, CH2CH2CH3), 2.7 (s, 3H, CH3), 7.2-7.9 (m, 8H, ArH), 8.7 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 348 (M+). Anal. Calcd. for C21H24N4O: C, 72.38; H, 6.94; N, 16.08. Found: C, 72.40; H, 6.97; N, 16.11%.
2-(N′-Cyclohexylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS4)
Yield = 77%, mp 226-228°C; IR (KBr) cm− 1: 3328 (NH), 1679 (C = O), 1611 (C = N); 1H-NMR (CDCl3): δ 0.8-1.7 (m, 10H, cyclohexyl), 1.9-2.0 (t, 3H, CH2CH3), 2.5-2.6 (q, 3H, CH2CH3), 7.4-3-8.0 (m, 8H, ArH), 8.5 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 360 (M+). Anal. Calcd. for C22H24N4O: C, 73.31; H, 6.71; N, 15.54. Found: C, 73.40; H, 6.70; N, 15.60%.
2-(N′-1-Phenylethylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS5)
Yield = 73%, mp 279-280°C; IR (KBr) cm− 1: 3316 (NH), 1685 (C = O), 1610 (C = N); 1H-NMR (CDCl3): δ 1.5-1.6 (t, 3H, CH2CH3), 1.8-1.9 (q, 3H, CH2CH3) 2.4 (s, 3H, CH3), 7.2-8.0 (m, 13H, ArH), 8.4 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 382 (M+). Anal. Calcd. for C24H22N4O: C, 75.37; H, 5.79; N, 14.64. Found: C, 75.39; H, 5.5.74; N, 14.68%.
2-(N′-2-Oxo-indolin-2-one-3-yl-idene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS6)
Yield = 74%, mp 216-218°C; IR (KBr) cm− 1: 3279 (NH), 1687 (C = O), 1610 (C = N); 1H-NMR (CDCl3): 1.3-1.4 (t, 3H, CH2CH3), 1.9-2.0 (q, 3H, CH2CH3), 7.1-8.0 (m, 12H, ArH), 8.3 (br s, 1H, NH, D2O Exchangeable), 8.8 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 409 (M+). Anal. Calcd. for C24H19N5O2: C, 70.40; H, 4.68; N, 17.10. Found: C, 70.45; H, 4.71; N, 17.13%.
2-(N′-Benzylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS7)
Yield = 77%, mp 241-242° C; IR (KBr) cm− 1: 3310 (NH), 1685 (C = O), 1613 (C = N); 1H-NMR (CDCl3): δ 1.8-1.9 (t, 3H, CH2CH3), 2.2-2.3 (q, 3H, CH2CH3), 6.1 (s, 1H, CH), 7.2-8.1 (m, 13H, ArH), 8.7 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 368 (M+). Anal. Calcd. for C23H20N4O: C, 74.98; 5.47, 15.20. Found: C, 74.94; H, 5.39; N, 15.21%.
2-(N′-(2-Chloro-benzylidene-hydrazino))-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS8)
Yield = 78%, mp 286-287°C; IR (KBr) cm− 1: 3290 (NH), 1681 (C = O), 1610 (C = N); 1H-NMR (CDCl3): δ 1.1-1.2 (t, 3H, CH2CH3), 2.5-2.6 (q, 3H, CH2CH3), 6.4 (s, 1H, CH), 7.1-8.1 (m, 12H, ArH), 8.6 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 402 (M+). Anal. Calcd. for C23H19N4OCl: C, 68.57; H, 4.75; N, 13.91. Found: C, 68.59; H, 4.71; N, 13.90%.
2-(N′-(4-Chloro-benzylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS9)
Yield = 77% yield, mp 239-240°C; IR (KBr) cm− 1: 3243 (NH), 1679 (C = O), 1610 (C = N); 1H-NMR (CDCl3): δ 1.8-1.9 (t, 3H, CH2CH3), 2.1-2.2 (q, 3H, CH2CH3), 6.0 (s, 1H, CH), 7.3-8.2 (m, 12H, ArH), 8.8 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 402 (M+). Anal. Calcd. for C23H19N4OCl: C, 68.57; H, 4.75; N, 13.91. Found: C, 68.63; H, 4.70; N, 13.87%.
2-(N′-(2-Nitro-benzylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS10)
Yield = 81%, mp 221-222°C; IR (KBr) cm− 1: 3041 (NH), 1686 (C = O), 1615 (C = N); 1H-NMR (CDCl3): δ 1.6-1.7 (t, 3H, CH2CH3), 2.1-2.2 (q, 3H, CH2CH3), 6.2 (s, 1H, CH), 7.3-8.2 (m, 12H, ArH), 8.5 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 413 (M+). Anal. Calcd. for C23H19N5O3: C, 66.82; H, 4.63; N, 16.93. Found: C, 66.85; H, 4.68; N, 16.91%.
2-(N′-(4-Nitro-benzylidene-hydrazino))-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS11)
Yield = 71%, mp 263-264°C; IR (KBr) cm− 1: 3263 (NH), 1689 (C = O), 1615 (C = N); 1H-NMR (CDCl3): δ 1.2-1.3 (t, 3H, CH2CH3), 2.4-2.5 (q, 3H, CH2CH3), 6.2 (s, 1H, CH), 7.2-8.1 (m, 12H, ArH), 8.6 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 413 (M+). Anal. Calcd. for C23H19N5O3: C, 66.82; H, 4.63; N, 16.93. Found: C, 66.81; H, 4.66; N, 16.97%.
2-(N′-(4-Methoxy-benzylidene-hydrazino))-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS12)
Yield = 79%, mp 229-230°C; IR (KBr) cm− 1: 3381 (NH), 1680 (C = O), 1610 (C = N); 1H-NMR (CDCl3): δ 1.4-1.5 (t, 3H, CH2CH3), 2.0-2.1 (q, 3H, CH2CH3), 3.2 (s, 3H, OCH3), 6.6 (s, 1H, CH), 7.0-7.9 (m, 12H, ArH), 8.5 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 398 (M+). Anal. Calcd. for C24H22N4O2: C, 72.34; H, 5.56; N, 14.06. Found: C, 72.37; H, 5.59; N, 14.04%.
2-(N′-(2-Methyl-benzylidene-hydrazino))-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS13)
Yield = 77%, mp 208-209°C; IR (KBr) cm− 1: 3327 (NH), 1689 (C = O), 1610 (C = N); 1H-NMR (CDCl3): δ 1.7-1.8 (t, 3H, CH2CH3), 2.5-2.6 (q, 3H, CH2CH3), 2.9 (s, 3H, CH3), 6.2 (s, 1H, CH), 7.1-8.0 (m, 12H, ArH), 8.3 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 382 (M+). Anal. Calcd. for C24H22N4O: C, 75.35; H, 5.80; N, 14.66. Found: C, 75.41; H, 5.78; N, 14.69%.
2-(N′-(4-Methyl-benzylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS14)
Yield = 74%, mp 261-263°C; IR (KBr) cm− 1: 3274 (NH), 1690 (C = O), 1615 (C = N); 1H-NMR (CDCl3): δ 1.3-1.4 (t, 3H, CH2CH3), 2.1-2.2 (q, 3H, CH2CH3), 2.6 (s, 3H, CH3), 6.4 (s, 1H, CH), 7.0-8.0 (m, 12H, ArH), 8.5 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 382 (M+). Anal. Calcd. for C24H22N4O: C, 75.35; H, 5.80; N, 14.66. Found: C, 75.33; H, 5.81; N, 14.67%.
2-(N′-Phenyl-benzylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS15)
Yield = 79%, mp 238-239°C; IR (KBr) cm− 1: 3278 (NH), 1690 (C = O), 1613 (C = N); 1H-NMR (CDCl3): δ 1.5-1.6 (t, 3H, CH2CH3), 2.3-2.4 (q, 3H, CH2CH3), 3.1 (s, 3H, OCH3), 7.1-8.3 (m, 18H, ArH), 8.6 (br s, 1H, NH, D2O Exchangeable); MS (m/z): 444 (M+). Anal. Calcd. for C29H24N4O: C, 78.35; H, 5.44; N, 12.60. Found: C, 78.41; H, 5.47; N, 12.61%.
Pharmacology
The synthesized compounds were evaluated for analgesic, anti-inflammatory, ulcerogenicindex and antimicrobial activities. Student t-test was performed to ascertain the significance of all the exhibited activities. The test compounds and the standard drugs were administered in the form of a suspension (1% carboxymethyl cellulose as a vehicle) by oral route of administration for analgesic and anti-inflammatory. For ulcerogenicity studies the drug were administrated by intraperitoneally as suspension in 10% v/v Tween 80. Each group consisted of six animals. The animals were procured from the Tetrex Biological Center, Madurai, India, and were maintained in colony cages at 25 ± 2°C, relative humidity of 45-55%, under a 12 h light and dark cycle; they were fed standard animal feed. All the animals were acclimatized for a week before use. The Institutional Animal Ethics committee has approved the protocol adopted for the experimentation of animals.
Analgesic activity
Test for analgesic activity was performed by tail-flick technique [Citation14,15] using Swiss albino mice (25-35 gm) of either sex selected by random sampling technique. Diclofenac sodium at a dose level of 10 mg/kg and 20 mg/kg was administered orally as reference drug for comparison. The test compounds at two dose levels (10 and 20 mg/kg) were administered orally. The reaction time was recorded at 30 min, 1, 2 and 3 h after the treatment, and cut-off time was 10. The percent analgesic activity (PAA) was calculated by the following formula,
where T1 is the reaction time (s) before treatment, and T2 is the reaction time (s) after treatment.
Anti-inflammatory activity
Anti-inflammatory activity was evaluated by carrageenan-induced paw oedema test in Albino rats of Wistar strain [Citation16]. Diclofenac sodium 10 and 20 mg/kg was administered as a standard drug for comparison. The test compounds were administered at two dose levels (10 mg & 20 mg/kg). The paw volumes were measured using the mercury displacement technique with the help of a plethysmograph immediately before and 30 min, 1, 2 and 3 h after carrageenan injection. The percent inhibition of paw oedema was calculated using the following formula
Where x is the mean paw volume of rats before the administration of carrageenan and test compounds or reference compound (test group), a is the mean paw volume of rats after the administration of carrageenan in the test group (drug treated), b is the mean paw volume of rats after the administration of carrageenan in the control group, y is the mean paw volume of rats before the administration of carrageenan in the control group.
Evaluation of ulcerogenicity index
Ulceration in rats was induced as described by Goyal et al [Citation17]. Albino rats of Wistar strain (150-200 g) of either sex were divided into various groups each of six animals. The control groups of animals were administered only with 10% v/v Tween 80 suspension intraperitonially. One group was administered with Aspirin (German Remedies) intraperitoneally in a dose of 200 mg/kg once daily for three days. The remaining group of animals was administered with test compounds intraperitoneally in a dose of 20 mg/kg. On fourth day, pylorus was ligated as per the method of Shay et al [Citation18]. Animals were fasted for 36 h before the pylorus ligation procedure. Four hours after the ligation, animals were sacrificed. The stomach was removed and opened along Table the greater curvature. Ulcer index was determined by the method of Ganguly and Bhatnagar [Citation19] and recorded in .
Statistical analysis
Statistical analysis of the biological activity of the synthesized compounds on animals was evaluated using a one-way analysis of variance (ANOVA). In all cases, post-hoc comparisons of the means of individual groups were performed using Tukey's test. A significance level of P < 0.05 denoted significance in all cases. All values are expressed as mean ± SD (standard deviations). For statistical analysis we have used GraphPad Prism 3.0 version. (GraphPad Prism 3.0 version, GraphPad Software, Inc.11452 El Camino Real, #215, San Diego, CA 92130 USA).
Results and discussion
Synthesis and characterization of AS1-AS 15
The key intermediate 3-(4-ethylphenyl)-2-thioxo-2,3-dihydro-1H-quinazolin-4-one 4 was synthesized by reacting 4-ethyl aniline (1) with carbon disulphide and sodium hydroxide in DMSO to give sodium dithiocarbamate, which was methylated with dimethyl sulphate to afford the dithiocarbamic acid methyl ester (2) (Scheme ). Compound 2 on reflux with methyl anthranilate (3) in ethanol yielded the desired 3-(4-ethylphenyl)-2-thioxo-2,3-dihydro-1H-quinazolin-4-one (4) via the thiourea intermediate in good yield (81%). The product obtained was cyclic and not an open chain thiourea 3a. It was confirmed by IR spectra of compound 4 showed intense peaks at 3210 cm-1 for cyclic thio urea (NH), 1690 cm− 1 for carbonyl (C = O) and 1218 cm− 1 for thioxo (C = S) stretching. 1H NMR spectra of 4 showed a triplet at δ 1.3-1.4 ppm due to CH3 group; a quartet at δ 2.1-2.2 due to CH2 and a multiplet at δ 7.3-8.0 ppm for aromatic (8H) protons and a broad singlet at δ 10.1 ppm indicating the presence of NH. Data from the elemental analyses were found to be in conformity with the assigned structure. Further the molecular ion recorded in the mass spectra is also in agreement with the molecular weight of the compound.
Scheme 1 Synthesis of 3-(2-ethylphenyl)-2-substitutedamino-3H-quinazolin-4-ones. Reagents and conditions: (a) DMSO, rt, 30 min; (b) (CH3)2SO4, 5-10°C, 2 h; (c) Methyl anthranilate (3), K2CO3, ethanol reflux for 22 h; (d) 2% alcoholic NaOH, (CH3)2SO4, rt, 1 h; (e) NH2NH2, K2CO3, ethanol reflux for 39 h; (f) (R2R1)CO; CH3COOH reflux, 36 h.
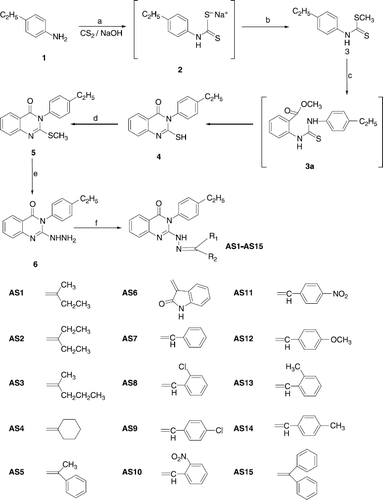
The 3-(4-ethylphenyl)-2-methysulfanyl-3H-quinazolin-4-one 5 was obtained by dissolving 4 in 2% alcoholic sodium hydroxide solution and methylating with dimethyl sulphate with stirring at room temperature. The IR spectra of 5 showed disappearance of NH and C = S stretching signals of cyclic thiourea. It showed a peak for carbonyl (C = O) stretching at 1681 cm-1. The 1H NMR spectra of compound 5 showed triplet at δ 1.5-1.6 ppm due to CH3; a quartet at δ 2.3-2.4 due to CH2; a singlet at δ 2.7 ppm SCH3 and multiplet at δ 7.2-7.8 ppm was observed for aromatic (8H) protons. Data from the elemental analyses and molecular ion recorded in the mass spectra further confirmed the assigned structure.
Nucleophilic displacement of methylthio group of 5 with hydrazine hydrate was carried out using ethanol as solvent to afford 3-(4-ethylphenyl)-2-hydrazino-3H-quinazolin-4-one 6. The long duration of reaction (39 h) required might be due to the presence of bulky aromatic ring at position 3, which might have reduced the reactivity of quinazoline ring system at C-2 position. The formation of 6 was confirmed by the presence of NH and NH2 signals at 3362-3281 cm− 1 in the IR spectra. It also showed a peak for carbonyl (C = O) at 1686 cm− 1. The 1H NMR spectra of the compound 6 showed triplet at δ 1.6-1.7 ppm due to CH3 group; a quartet at δ 2.2-2.3 due to CH2, broad singlet at δ 5.3 ppm and 9.8 ppm due to NH2 and NH respectively, a multiplet at δ 7.4-8.1 ppm was observed for aromatic (8H) protons. Data from the elemental analyses were found to be in conformity with the assigned structure. Further the molecular ion recorded in the mass spectra is also in agreement with the molecular weight of the compound.
The title compounds 3-(4-ethylphenyl)-2-substituted amino-3H-quinazolin-4-ones AS1-AS15 were obtained by the condensation of amino group of 2-hydrazino-3-(4-ethyl phenyl)-3H-quinazolin-4-one (6) with a variety of aldehydes and ketones. The formation of title product is indicated by the disappearance of peak due to NH2 of the starting material in IR and 1H-NMR spectrum of all the compounds AS1-AS15. The IR and 1H-NMR spectrum these compounds showed the presence of peaks due to (N = CR1R2) carbonyl (C = O), NH and and Aryl groups. The mass spectrum of these compounds showed molecular ion peaks corresponding to their molecular formula and are in confirmed with the assigned structure. In mass spectrum of compounds AS1-AS15 a common peak at m/z 144 corresponding to quinazolin-4-one moiety appeared in all mass spectra. Elemental (C, H, N) analyses satisfactorily confirmed elemental composition and purity of the synthesized compounds.
Pharmacology
Analgesic activity
Evaluation of analgesic activity was performed by the tail-flick technique [Citation14,15] using Swiss albino mice. The results of analgesic testing inidcate that the test compounds exhibited moderate anagesic activity at 30 minutes of reaction time and an increase in activity at 1 h which reached a peak level at 2 hours. Decline in activity was observed at 3 hours (). Compound AS1 with 1-methylpropylidene substituent showed good activity; with the increased lipophilicity (1-ethylpropylidene group, compound AS2) showed increase in activity. Replacement of 1-ethylpropylidene group with its isomer 1-methylbutylidene group (compound AS3) retains the activity. Replacement of C-2 alkyl chain with a cycloalkyl group and an aralkyl group (compounds AS4 and AS5 respectively) leads to a moderate decrease in activity. Placement of aryl group at the N-3 position (compounds AS6, AS7 and AS13-AS15) also results in decreasing the activity. Placement of electron withdrawing group at N-3 aryl ring (compounds AS8-AS12) leads to further decrease of activity. Compound 2-(1-ethylpropylidene)-hydrazino-3-(4-ethylphenyl)-quinazolin-4(3H)-one (AS2) emerged as the most active analgesic agent and it is moderately more potent when compared to the reference standard diclofenac.
Table I. Analgesic activity of AS1-AS15.
Anti-inflammatory activity
Anti-inflammatory activity was evaluated by the carragenenan-induced paw edema test in Albino rats of Wistar strain [Citation16]. The anti-inflammatory activity data () indicated that all the test compounds protected rats from carrageenan-induced inflammation moderately at 30 minutes of reaction time with increased activity at 1 hours that reached a peak level at 2 h. Decline in activity was observed at 3 hours. The compound 2-(1-ethylpropylidene)-hydrazino-3-(4-ethylphenyl)-quinazolin-4(3H)-one (AS2) showed the most potent anti-inflammatory activity of the series and it is moderately more potent when compared to the reference standard diclofenac sodium.
Table II. Anti-inflammatory activity of synthesized compounds (AS1-AS15).
Ulcer index
The ulcer index of the test compounds () reveal that the compounds with aliphatic substituents (compounds AS1-AS4) showed negligible ulcer index, whereas aryl substituents (compounds AS5-AS7 and AS13-AS15) exhibited little increase in ulcer index and the aryl substituents containing electron withdrawing groups (compounds AS8-AS12) exhibited higher ulcer index over other test compounds. When compared to the reference standards aspirin (ulcer index 1.73 ± 0.41) and diclofenac (ulcer index 1.65 ± 0.59) the test compounds exhibited about 35% to 50% of the ulcer index of the reference standards. The compound 2-(N′-3-Pentylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS2) and 2-(N′-2-Pentylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS3) exhibited least ulcer index (0.51 ± 1.39 and 0.48 ± 1.61 respectively) among the test compounds which is about one third of the ulcer index of reference standards aspirin and diclofenac. The compound 2-(N′-(4-Chloro-benzylidene-hydrazino)-3-(4-ethylphenyl)-3H-quinazolin-4-one (AS9) showed the highest ulcer index (0.83 ± 1.55) among the test compounds which is about 50% of the ulcer index of reference standards aspirin and diclofenac.
Table III. Evaluation of ulcerogenicity index.
In our earlier studies [Citation10–13] we observed that the presence of alkyl groups exhibited more analgesic and anti-inflammatory activities over aryl groups at the N-3 position. Hence in the C-2 position also we made a substitution in such a way to increase lipophilicity of the molecule. The placement of such a group enhanced the analgesic and anti-inflammatory activities. The most active compound of the C-2 phenyl series 1-diethyl-3-(2-phenyl quinazolin-3-yl-4(3H)-one) thiourea (, I) showed 44% & 58% analgesic and 38% & 53% anti-inflammatory activity at the dose of 10 & 20 mg/kg respectively, at the reaction time of 2 hours [Citation10]. Whereas the C-2 methyl series lead molecule 1-pyrrolidinyl-3-(2-methyl quinazolin-3-yl-4(3H)-one) thiourea (, II) exhibited 50% & 65% analgesic and 44% & 60% anti-inflammatory activity at the dose of 10 & 20 mg/kg respectively at the reaction time of 2 hours [Citation11]. Introduction of sulphur atom at C-2 position in the above series i.e. by placing methylthio group at C-2 position [Citation12] compound 1-diethyl-3-(2-methylthio quinazolin-3-yl-4(3H)-one) thiourea (, III) exhibited 56% & 67% analgesic activity, 40% & 62% anti-inflammatory activity at 10 & 20 mg/kg respectively at the reaction time of 2 hours. Results of analgesic and anti-inflammatory activities of the present series showed that moderate enhancement of activity, compound AS2 exhibited 68% & 80% analgesic activity at 10 and 20 mg/kg dose level respectively at the reaction time of 2 hours. The compound AS2 showed 53% & 69% anti-inflammatory activity at the dose of 10 and 20 mg/kg respectively at the reaction of 2 hours. Interestingly these compounds showed one third of the ulcer index of the reference NSAID's aspirin and diclofenac. Hence this series could be developed as a novel class of analgesic and anti-inflammatory agents.
References
- J Van Ryn, and RM Botting. (1995). New insights into the mode of action of anti-inflammatory drugs. Inflamm Res 44:1–10.
- J Van Ryn, G Trummlitz, and M Pairet. (2000). COX-2 selectivity and inflammatory processes. Curr Med Chem 7:1145–1161.
- J Van Ryn. (1971). Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature: New Biology 231:232–235.
- M Beuck. (1999). Nonsteroidal Anti-inflammatory drugs: A new generation of Cyclooxygenase inhibitors. Angew Chem Int Ed 38:631–633.
- V Alagarsamy, R Revathi, S Vijayakumar, and KV Ramseshu. (2003). Synthesis and pharmacological investigation of some novel 2,3-disubstituted quinazolin-4(3H)-ones as analgesic and anti-inflammatory agents. Pharmazie 58:233–236.
- V Alagarsamy, A Thangathirupathy, SC Mandal, S Rajasekaran, S Vijayakumar, R Revathi, J Anburaj, S Arunkumar, and S Rajesh. (2006). Pharmacological evaluation of 2-substituted (1,3,4) thiadiazolo quinazolines. Indian J Pharm Sci 68:108–111.
- AE Abdel-Rahman, EA Bakhite, and EA Al-Taifi. (2003). Synthesis and antimicrobial testing of some new S-substituted-thiopyridines, thienopyridines, pyridothienopyrimidines and pyridothienotriazines. Pharmazie 58:372–377.
- RV Chambhare, BG Khadse, AS Bobde, and RH Bahekar. (2003). Synthesis and preliminary evaluation of some N-[5-(2-furanyl)-2-methyl-4-oxo-4H-thieno[2,3-d]pyrimidin-3-yl]-carboxamide and 3-substituted-5-(2-furanyl)-2-methyl-3H-thieno[2,3-d]pyrimidin-4-ones as antimicrobial agents. Eur J Med Chem 38:89–100.
- NA Santagati, A Caruso, VMC Cutuli, and F Caccamo. (1995). Synthesis and pharmacological evaluation of thieno[2,3-d]pyrimidin-2,4-dione and 5H-pyrimido [5,4-b]indol-2,4-dione derivatives. Il Farmaco 50:689–695.
- V Alagarsamy, VR Solomon, G Vanikavitha, V Paluchamy, MR Chandran, AA Sujin, A Thangathiruppathy, S Amuthalakshmi, and R Revathi. (2002). Synthesis, analgesic, anti-inflammatory and antibacterial activities of some novel 2-phenyl-3-substituted quinazolin-4(3H) ones. Biol Pharm Bull 25:1432–1435.
- V Alagarsamy, G Murugananthan, and R Venkateshperumal. (2003). Synthesis, analgesic, anti-inflammatory and antibacterial activities of some novel 2-methyl-3-substituted quinazolin-4-(3H)-ones. Biol Pharm Bull 26:1711–1714.
- V Alagarsamy, R Rajesh, R Meena, S Vijaykumar, KV Ramseshu, and T Duraianandakumar. (2004). Synthesis, analgesic, anti-inflammatory and antibacterial activities of some novel 2-methylthio-3-substituted quinazolin-4(3H)-ones. Biol Pharm Bull 27:652–656.
- V Alagarsamy, V Muthukumar, N Pavalarani, P Vasanthanathan, and R Revathi. (2003). Synthesis, analgesic and anti-inflammatory activities of some novel 2,3-disubstituted quinazolin-4(3H)-ones. Biol Pharm Bull 26:557–559.
- SK Kulkarni. (1980). Heat and other physiological stress-induced analgesia: Catecholamine mediated and naloxone reversible response. Life Sci 27:185–188.
- RE D'Armour, and DL Smith. (1941). A method for determinating loss of pain sensation. J Pharm Exp Therap 72:74–78.
- CA Winter, EA Risely, and GW Nuss. (1962). Carregeenin induced oedema in hind paw of the rat as assay for anti-inflammatory drugs. Exp Biol Med 111:544–547.
- RK Goyal, A Chakrabarti, and AK Sanyal. (1985). The effect of biological variables on the anti-ulcerogenic effect of vegetable plantain banana. Planta Medica 29:85–88.
- M Shay, SA Komarov, D Fels, D Meranze, H Grunstein, and H Siplet. (1945). A simple method for the uniform production of gastric ulceration in the rats. Gastroenterol 5:43–61.
- AK Ganguly, and OP Bhatnagar. (1973). Effect of bilateral adrenalectomy on production of restraint ulcers in the stomach of albino rats. Can J Phys Pharmacol 51:748–750.