Abstract
The methanol extract of the rhizomes of Gloriosa superba Linn (Colchicaceae) and its subsequent fractions in different solvent systems were screened for antibacterial and antifungal activities. Excellent antifungal sensitivity was expressed by the n-butanol fraction against Candida albicans and Candida glaberata (up to 90%) and against Trichophyton longifusus (78%) followed by the chloroform fraction against Microsporum canis (80%). In the antibacterial bioassay, the crude extract and subsequent fractions showed mild to moderate antibacterial activities. Chloroform fraction displayed highest antibacterial sensitivity against Staphylococcus aureous (88%) followed by the crude extract (59%). The total phenol content of the crude extract and fractions of the plant expressed no significant correlation with the antimicrobial activities.
Introduction
Considerable work have been done on various sources such as microorganisms, animals, and plants to design and develop new antimicrobial compounds for the treatment of microbial infections, both topical and systemic applications, as possible alternatives to chemically synthetic drugs [Citation1]. Literature reports and ethnobotanical records suggest that plants are the sleeping giants of the pharmaceutical industry [Citation2]. They may provide natural source of antimicrobial drugs that will/or provide novel or lead compounds that may be employed in controlling some infections globally. But scientific studies on the antimicrobial properties of plants and their components have been documented in the late 19th century [Citation3]. Clinical microbiologists have two reasons to be interested in the topic of antimicrobial plant extracts.
Finding healing powers in plants is an ancient idea and despite the remarkable progress in synthetic organic chemistry of the twentieth century, over 25% of prescribed medicines in industrialized countries derived directly or indirectly from plants. However, plants used in traditional medicine are still understudied, particularly in clinical microbiology [Citation4]. Recently, the biological control has gained tremendous importance over chemicals antimicrobials [Citation5], mostly because of the side effects of the synthetic products. Even commonly used food stuff contains a number of naturally occurring antimicrobial agents that help in the prevention of the food material from deterioration [Citation6]. The increasing prevalence of multidrug resistant strains of bacteria and the recent appearance of strains with reduced susceptibility to antibiotics raises the specter of untreatable bacterial infections and adds urgency to the search for new infection fighting strategies [Citation7]. Plant-based antimicrobials have a considerable safety profile and are not associated with many side effects when compare with synthetic products. Additionally, they have an enormous therapeutic potential to heal many infectious diseases [Citation8].
The plant Gloriosa superba Linn is commonly known as climbing lily (English) and Samp ki Bothi (Urdu) and belongs to the Colchicaceae family. It is a perennial herb, semi-woody herbaceous, branching climber, reaching approximately 5 metres in height. 1 to 4 stems arise from single V-shaped fleshy cylindrical tuber/rhizomes. The perianth segments, which are accrescent during anthesis and become reflexed, are striking in color, yellow proximally and at margins and dark red in the median portion [Citation9,10]. A native of tropical Africa and is now found growing naturally throughout much of tropical Asia including: India, Sri Lanka, Malaysia and Burma [Citation11].
In traditional medicine practiced, it's important medicinal used is as a source of colchicine, and both seed and rhizome are used for the treatment of gout [Citation12,13]. The juice of the leaves is used to kill head lice and also as an ingredient in arrow poisons, as well as antimosquito properties [Citation14]. The rhizomes also have antidotal properties to snakebite and in India it is commonly placed on windowsills to deter snakes Many cultures believe the species to have various magical properties [Citation15–17]. The crude extract and fractions expressed outstanding inhibition on lipoxygenase. Phytochemically, in addition to colchicine and gloriosine, Gloriosa superba also contains other alkaloid such as 3-desmethyl colchicine, β-lumicolchicine, N-Formyldesacetyl-colchicine, 2-desmethyl colchicine, and compounds like, chelidonic acid and salicylic acid [Citation18].
Materials and methods
Plant material
The Gloriosa superba Linn, as a whole plant was collected from Chatter Bagh, Islamabad (Pakistan) during the month of June 2007. Authentication of the plant material was done by the Botany department of the PCSIR Laborites Peshawar and a voucher specimen was deposited there.
Extraction
The shade dried plant material (rhizomes) was chopped into small pieces and finally pulverized into fine powder. The powdered plant material (9 Kg) was soaked in methanol with occasional shaking, at room temperature. After 15 days, the methanol soluble materials were filtered off. The filtrate was concentrated under vacuum at low temperature (40°C) using rotary evaporator. A crude extract (269 g) was obtained.
Fractionation
The crude methanol extract (967g) was suspended in distilled water (500 mL) and sequentially partitioned with n-hexane (3 × 500 mL), chloroform (3 × 500 mL), ethyl acetate (3 × 500 mL) and n-butanol (3 × 500 mL) to yield the n-hexane (123 g), chloroform (231 g), ethyl acetate (180g), n-butanol (203 g) and aqueous (161 g) fractions, respectively.
Antifungal bioassay
Antifungal activity of the crude extract and various fractions were evaluated by agar tube dilution method [Citation19]. The samples at concentrations of 24mg/mL were dissolved in the sterile (autoclaved) dimethyl sulfoxide (DMSO, Merck), which served as stock solution. Sabouraud dextrose agar (SDA, Sigma-Aldrich, Germany) was prepared by mixing 32.5 g sabouraud, 4% glucose agar and 4.0 g of agar-agar in 500 mL distilled water. It was then stirred with a magnetic stirrer to dissolve it. Then 4mL amount was dispensed into screw cap tubes, which were autoclaved at 120°C for 15 min and then cooled to 15°C. The non-solidified SDA media was poisoned with stock solution (66.6μL) giving the final concentration of 400μg of the extract per mL of SDA. Tubes were then allowed to solidify in the slanted position at room temperature. Each tube was inoculated with a piece (4 mm diameter) of inoculums removed from a seven days old culture of fungi for non-mycelial growth; an agar surface streak was employed. Other media supplemented with dimethyl sulfoxide (DMSO) and reference anti-fungal drugs served as negative and positive control respectively. Inhibition of fungal growth was observed after 7-days of incubation at 28 ± 1°C. Humidity (40–50%) was controlled by placing an open pan of water in the incubator. After the incubation period for 7-days, the test tubes were analyzed for the visible growth of the microorganisms.
Antibacterial bioassay
The crude extract and its various fractions in concentration of 3mg/mL were also screened against various human pathogens including Escherichia coli, Bacillus subtilis, Klebsiella pneumonae, Shigella flexenari, Staphylococcus aureous, and Salmonella typhi by agar well diffusion method [Citation19]. In this method, 10 mL aliquots of nutrients broth (Sigma-Aldrich, Germany) was inoculated with the test organism and incubated at 37°C for 24 h. Using a sterile pipette, 0.6 mL of the broth culture of the test organism was added to 60 mL of molten agar, which had been cooled to 45°C, mixed well and poured into a sterile Petri dish (for the 9 cm Petri dish, 0.2 mL of the culture was added to 20 mL of agar). Duplicate plates of each organism were prepared. The agar was allowed to set and harden and the required number of wells were dug in the medium using a sterile metallic cork borer ensuring proper distribution of the wells in the periphery and one in the center. Agar plugs were removed. Stock solutions of the test samples at a concentration of 1mg/mL were prepared in the sterile dimethyl sulfoxide (DMSO) and 100μL and 200 μL of each dilution was added in their respective wells. Control well received only 100 μL and 200μL of DMSO. Imipinem was used as standard drug (10μg / mL). The plates were left at room temperature for 2 h to allow diffusion of the samples then incubated face upwards at 37°C for 24 h. The diameter of the zones of inhibition was measured to the nearest mm (the well size also being noted).
Total phenol content determination
According the standard method of Folin and Denis [Citation20,21], the total phenol concentration of the crude extract and subsequent fractions of the Gloriosa superba Linn were determined. Briefly, each extract (10mg) was mixed with Folin-Denis reagent (5mL), Na2CO3 (20%, 10mL) and diluted by a factor 100 with distilled water. The resulting mixture was left at room temperature for 10 min after filtration and the absorbance was measured at 770 nm against the blank using Spectronic 20D (Milton Roy). The total phenol content of each extract and fraction was estimated by comparison with a standard curve generated using tannic acid.
Results and discussion
Traditional health care systems using medicinal plants can be recognized and used as a starting point for the development of novelties in drugs. For this purpose, crude methanol extract and subsequent fractions of Gloriosa superba Linn were screened for biological activities especially antibacterial and antifungal activities.
Anti-fungal activities of the crude extract and subsequent fractions of the Gloriosa superba Linn were evaluated for T. longifusus, C. albicans, Aspergilus flavus, Microsporum canis, C. glaberata and Aspergillus niger in comparison with miconazole and amphotericin-B. Growth in the medium containing the extract was determined by measuring the linear growth in mm and percentage growth was calculated with reference to the negative control. The antifungal results are displayed in . The crude extract and its various fractions showed good to excellent activity against T. longifusus. T. longifusus belongs to the genus Trichophyton, which is the causative agent of dermatophytosis and infects the hair, skin, and nails [Citation22,23]. Trichophyton species may cause invasive infections in immunocompromised hosts [Citation24].
Table I. Anti-fungal activities of crude extract and various fractions of Gloriosa superba Linn.
For T. longifusus, as shown in , crude extract showed (52%) inhibition, while chloroform fraction displayed excellent antifungal activity (67%). Both ethyl acetate and n-butanol fractions exhibited significant results, that is, (67%) and (78%) respectively. However, only (18%) antifungal activity was shown by the aqueous fraction. Crude extract and subsequent fractions showed outstanding activity against M. canis, which causes the infections of hair, skin and nail. Immunocompromised patients are also infected [Citation25–27]. Crude extract displayed (70%) activity; while chloroform fraction demonstrated outstanding (80%) antifungal activity, ethyl acetate fraction (70%), n-butanol fraction (75%) while aqueous fraction was devoted of any activity.
Figure 1. Antifungal activity of the crude extract and subsequent fractions of the Gloriosa superba Linn at 400 μg/ mL.
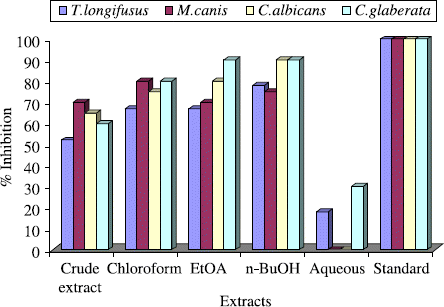
C. albicans and C. glaberata were extremely sensitive to Gloriosa superba Linn which are the main causative agents of invasive candidiasis including vaginal candidiasis [Citation28–30]. In case of C. albicans, crude extract exhibited (65%), chloroform friction (75%), ethyl acetate (80%) and n-butanol (90%) activity, while aqueous fraction was inactive. For C. glaberata, crude extract posses (60%), chloroform fraction (80%), ethyl acetate fraction (90%), n-butanol fraction (90%) and aqueous fraction (30%) activity. On the other hand, crude extract and subsequent fractions displayed no anti-fungal activity A. flavus and F. solani against in the assay.
The crude extract and subsequent fractions of Gloriosa superba Linn were also screened against various human pathogens including E. coli, B. subtilis, K. pneumonae, S. flexenari, S. aureous, P. aeruginosa and S. typhi. The results are presented in (). According to antibacterial bioassay, the crude extract and various fractions of Gloriosa superba Linn expressed excellent activity against S. aurous. The n-butanol fraction displayed the highest antibacterial activity (88%) against S. aurous in the assay among the tested pathogen. The crude extract exhibited (59%), chloroform fraction (52%), ethyl acetate fraction (26%) while aqueous fraction was inactive in the assay ().
Table II. Antibacterial activities of crude extract and various fractions of Gloriosa superba Linn.
Figure 2. Antibacterial activity of the crude extract and subsequent fractions of the Gloriosa superba Linn against S. aurous at 100 μg/mL.
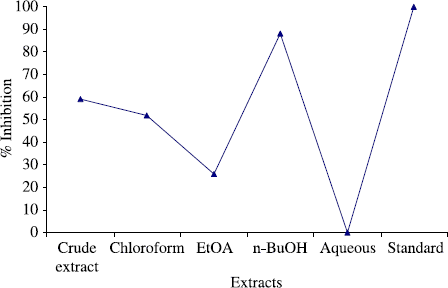
The antibacterial activity of the plant was low to moderate in antibacterial bioassay for S. flexenari. It has been observed that crude extract exhibited (42%), chloroform fraction (33%), ethyl acetate (42%), n-butanol fraction (50%) and aqueous fraction (46%) activity. Plant also exhibited low activity against B. subtilis. Crude extract exhibited (13%), while both chloroform and ethyl acetate fraction demonstrated (17%) activity for each and aqueous fraction displayed (26%) activity. The crude extract and subsequent fractions showed no zone of inhibition against, S. typhi, E. coli, K. pneumonae and P. aeruginosa.
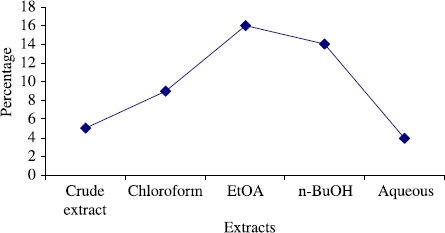
The total phenol content of the crude extract and subsequent fractions of the Gloriosa superba Linn were also determined (), in order to analyze any possible correlation between the antimicrobial activities and total phenol content of the plant fractions. It is well documented in literature that the phenols have significant antimicrobial activities and have a broad spectrum against both bacteria and fungi [Citation31–33]. In our present investigation, there was no correlation between the antimicrobial activity and phenol content. Therefore, it can be assumed that some other mechanism is responsible for the antifungal and antibacterial effects of this plant.
Figure 3. Total phenol content of the crude extract and subsequent fractions of Gloriosa superba Linn.
Results of present investigation, revealed that this plant species has excellent antifungal sensitivity against C. albicans, C. glaberata, T. longifusus and M. canis and significant antibacterial activity against S. aureous. Therefore, it could be a natural antifungal agent against these pathogens in different infections. However, further phytochemical and pharmacological investigation of the plant is required to explore the exact mechanism of antimicrobial activity and also to confirm other ethno-botanical uses in indigenous system of medicine.
References
- Dagmar Janovska, Katerina, Kubikova, and Ladislav Kokoska. (2003). Screening for antimicrobial activity of some medicinal plants species of traditional Chinese medicine. Czech J Food Sci 21:107–110.
- K Hostettmann, and M Hamburger. (1991). Phytochem. 30 (12):3864–3874.
- LL Zaika. (1975). Spices and herbs: Their antimicrobial activity and its determination. J Food Safety 9:97–118.
- GC Kirby. (1996). Medicinal plants and the control of parasites. Trans Roy Soc Trop Med Hyg 90:605–609.
- I von der Weid, DS Alviano, ALS Santos, RMA Soares, CS Alviano, and L Seldin. (2003). J App Microbiol 95:1143–1151.
- LA Shelef, OA Naglik, and DW Bogen. (1980). Sensitivity of some common food-borne bacteria to the spices sage, rosemary and allspice. J Food Sci 45:1042–1044.
- K Sieradzka, RB Robers, SW Harber, and A Tomasz. (1999). The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med 340:517–523.
- MW Iwu, AR Duncan, and CO Okunji. New antimicrobials of plant origin. J Janick, editor. Perspectives on new crops and new uses. Alexandria, VA: ASHS Press; (1999). p 457–462.
- AC Smith. (1979). Flora vitiensis nova: A new flora of Fiji. Lawai, Kauai, Hawaii. National Tropical Botanical Garden. 1:141–142.
- A Huxleyed-in-chief. The Royal Horticultural Society dictionary of gardening. vol. 2. London: MacMillan Press; (1992).
- DMA Jayaweera. Medicinal plants used in Ceylon. Colombo: National Science Council of Sri Lanka; (part 3)(1982).
- , and Anon. The wealth of India: A dictionary of raw material and industrial products. vol. 4. Delhi: CSIR; (1956). p 139–140.
- DS Bhakuni, and Sudha Jain. In: KL Chadha, and Rajendra Gupta. Advances in Horticulture. vol. 11. Delhi: Malhotra Publishing House; (1995). p 98–99.
- W Choochote, K Rongsriyam, B Pitasawat, A Jitpakdi, E Rattanachanpichai, A Junkum, B Tuetun, and P Chaiwong. (2004). Evaluation of the colchicine-like activity of Gloriosa superba-extracted fractions for mosquito (Diptera: Culicidae) cytogenetic study. J Med Entomol 41:672–676.
- JM Watt, and MG Breyer-Brandwijk. The medicinal and poisonous plants of Southern and Eastern Africa. Edinburgh, E. & S. Livingstone; (1962).
- HD Neuwinger. African ethnobotany. Poisons and drugs. Chemistry, pharmacology, toxicology. English translation by A Porter. Weinheim: Chapman & Hall; (1994).
- HM Burkill. The useful plants of West Tropical Africa. vol. 3. 2nd ed.Royal Botanic Gardens, Kew; (1995).
- JA Duke. Handbook of medicinal herbs. USA: CRC Press; (1985).
- Choudhary MI Atta-ur-Rehman, and Thomsen. Manual of Bioassay Techniques for Natural Product Research. Amsterdam: Harward Academic Press; (1991). p 82–84.
- O Folin, and Denis. (1912). J Biol Chem 12:243–339.
- O Folin, and Denis. (1915). J Biol Chem 22:305–308.
- R Aly, RJ Hay, A Del Palacio, and R Galimberti. (2000). Epidemiology of tinea capitis. Med Mycol 38:183–188.
- S Atman, TS Haroon, I Hussain, MA Bokhari, and K Khurshid. (2001). Tinea unguium in Lahore, Pakistan. Med Mycol 39:177–180.
- RF Squeo, R Beer, D Silvers, I Weitzman, and M Grossman. (1998). Invasive Trichophyton rubrum resembling blastomycosis infection in the immunocompromised host. J Am Acad Dermatol 39:379–380.
- R Aly. (1999). Ecology, epidemiology and diagnosis of tinea capitis. Pediat Inf Dis J 18:180–185.
- L Collier, A Balows, and M Sussman. Topley and Wilson's Microbiology and Microbial Infections. vol. 4. 9th ed.. , Arnold, London, Sydney, Auckland, New York: (1998) .
- P Garcia-Martos, J Gene, M Sole, J Mira, R, and J Guarro. (1999). Case of onychomycosis caused by Microsporum racemosum. J Clin Microbiol 37:258–260.
- Stephen P Saville, Anna L Lazzell, Alexander P Bryant, Angelika Fretzen, Alex Monreal, Erik O Solberg, Carlos Monteagudo, Jose L Lopez-Ribot, and G Todd Milne. (2006). Inhibition of filamentation can be used to treat disseminated candidiasis. Antimicrob Agents Chemother 50:3312–3316.
- A Viudes, J Peman, E Canton, P Ubeda, JL Lopez-Ribot, and M Gobernado. (2002). Candidemia at a tertiary-care hospital: Epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis 21:767–774.
- K Tanju, G Birsay, and U Banu. (2007). Phospholipase activity of Candida albicans isolates from patients with denture stomatitis: The influence of chlorhexidine gluconate on phospholipase production. Arch Oral Biol 52:691–696.
- Melissa A Mundo, Olga I Padilla-Zakour, and Randy W Worobo. (2004). Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. International J Food Microbiol 97:1–8.
- V Alavandi, KK Vijayan, TC Santiago, M Poornima, KP Jithendran, SA Ali, and JJS Rajan. (2004). Evaluation of Pseudomonas sp. PM 11 and Vibrio fluvialis PM 17 on immune indices of tiger shrimp, Penaeus monodon. Fish Shellfish Immunol 17:115–120.
- V Kuete, JG Tangmouo, V Penlap Beng, and FND Ngounound. (2006). Lontsi, Antimicrobial activity of the methanolic extract from the stem bark of tridesmostemon omphalocarpoides (Sapotaceae). J Ethnopharmacol 104:5–11.
- Haroon Khan, Murad Ali Khan, and Iqbal Hussan. Enzymes inhibition activities of the rhizomes of Gloriosa superba Linn (Colchicaceae). J Enz Inhib Med Chem [accepted]
