Abstract
A series of 3-amino- and polyaminosterol analogues of squalamine and trodusquemine were synthesized and evaluated for their in vitro antimicrobial properties against human pathogens. The activity was highly dependent on the structure of the different compounds involved and the best results were obtained with aminosterol derivatives 4b, 4e, 8b, 8e and 8n exhibiting minimum inhibitory concentrations (MICs) against yeasts, Gram positive and Gram negative bacteria at average concentrations of 3.12–12.5 μM.
Introduction
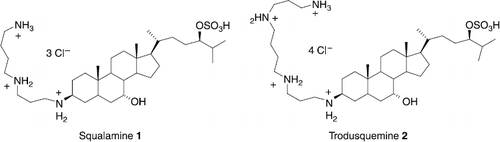
Emergence of multidrug-resistant microorganisms such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus has prompted efforts to develop new classes of antibiotics.[Citation1–5] In the search for novel host defense agents, squalamine 1 and trodusquemine 2 (Scheme ) were identified as the first aminosterols from the dogfish shark, squalus acanthias, exhibiting potent antimicrobial and antiangiogenic activities and representing an attractive way face to the increase of resistance that alters the activity of usual antibiotics on bacterial pathogens.[Citation6–12] These two derivatives possess an invariant steroid skeleton with a trans AB ring junction, a cholestane-related sulfated side chain and a flexible polyamino hydrophilic chain (spermidine and spermine group respectively) linked to the hydrophobic unit at C-3 position. Polyamines are essential for the growth and function of normal cells.[Citation13–17] They interact with various macromolecules, both electrostatically and covalently and, as a consequence, have a variety of cellular effects. The complexity of polyamine metabolism and the multitude of compensatory mechanisms that are invoked to maintain polyamine homeostasis argue that these amines are critical to cell survival. Recently, Katsu et al. examined the structure-activity relationship between functionalized polyamines and the outer membranes of Gram-negative bacteria and demonstrated that lipophilic moieties and a number of amino groups in polyamines were important for permeabilisation of such an outer membrane.[Citation18] In the same context, various polyamine conjugated to cholesterol, cholenic acid and bile acids have been reported possessing significant antimicrobial activities and reduced hemolytic potentials. [Citation19ndash;23] Nevertheless, all these synthesis were performed in numerous steps and low overall chemical yields. Since hydrophobicity of the sterol backbone and length and the cationic charge of the side chains appeared to be critical determinants of activity, we have envisioned the synthesis of new polyamino squalamine mimics. Recently, we have reported a new and efficient methodology for the synthesis of various secondary amines involving a titanium reductive amination reaction.[Citation24,25] In continuation of our work, we report herein the evaluation of the antimicrobial activities of various new 3-amino and polyaminosterol analogues of squalamine and trodusquemine.
Materials and Methods
All solvents were purified according to reported procedures, and reagents were used as commercially available. Methanol, ethyl acetate, dichloromethane, ammonia and petroleum ether (35–60 °C) were purchased from SDS and used without further purification. Column chromatography was performed on SDS silica gel (70–230 mesh). 1H NMR and 13C NMR spectra were recorded in CDCl3 on a Bruker AC 300 spectrometer working at 300 MHz and 75 MHz, respectively (the usual abbreviations are used: s: singlet, d: doublet, t: triplet, q: quadruplet, m: multiplet). Tetramethylsilane was used as internal standard. All chemical shifts are given in ppm.
General Procedure for the Titanium–mediated reductive amination reaction of 3β-(1,2-diaminoethane)-4-cholestene 4c
A mixture of 4-cholesten-3-one 3 (300 mg, 0.78 mmol), titanium(IV)isopropoxide (302 μL, 1.03 mmol) and 1,2-diaminoethane (140 mg, 2.34 mmol) in absolute methanol (5 mL) was stirred under argon at room temperature for 12 h. Sodium borohydride (29 mg, 0.78 mmol) was then added at − 78°C and the resulting mixture was stirred for an additional 2 h. The reaction was then quenched by adding water (1 mL) and stirring was maintained at room temperature for 20 min. The resulting inorganic precipitate was filtered off over a pad of Celite and washed with Et2O and ethylacetate. The combined organic extracts were dried over Na2SO4, filtered and concentrated in vacuo to afford the expected crude amine 4c. Subsequent purification by flash chromatography on silicagel (eluent: CH2Cl2/MeOH/NH4OH(32%), 7:3:1) led to a pale yellow solid in 60% yield; –1H NMR (300 MHz, CDCl3): δ = 5.14 (s, 1H), 3.29–2.62 (m, 5H), 2.32–0.55 (m, 46H). –13C NMR (75 MHz, CDCl3): δ = 146.32, 122.05, 56.03, 54.45, 49.37, 48.82, 42.27, 41.80, 39.72, 39.30, 37.30, 36.24, 35.97, 35.76, 35.59, 33.00, 32.28, 28.01, 27.78, 26.92, 24.04, 23.67, 22.63, 22.37, 20.97, 18.92, 18.47, 11.78.
All the 3-amino-or polyaminosterol derivatives (Scheme ) were prepared by procedures similar to that described above and all the NMR analysis are in accordance with the expected data. The obtained MS results of the corresponding products were as follows: 4b MS (ESI+) m/z 400.1 (100%, [M + H]+). 4c MS (ESI+) m/z 429.0 (100%, [M + H]+). 4d MS (ESI+) m/z 443.6 (100%, [M + H]+). 4e MS (ESI+) m/z 457.4 (100%, [M + H]+). 4f MS (ESI+) m/z 499.6 (100%, [M + H]+). 4g MS (ESI+) m/z 485.6 (100%, [M + H]+). 4h MS (ESI+) m/z 471.5 (100%, [M + H]+). 4i MS (ESI+) m/z 513.6 (100%, [M + H]+). 4k MS (ESI+) m/z 541.5 (100%, [M + H]+). 4l MS (ESI+) m/z 569.5 (100%, [M + H]+). 4m MS (ESI+) m/z 571.6 (100%, [M + H]+). 4n MS (ESI+) m/z 468.5 (100%, [M + H]+). 4p MS (ESI+) m/z 428.3 (100%, [M + H]+). 6b MS (ESI+) m/z 402.4 (100%, [M + H]+). 6c MS (ESI+) m/z 431.4 (100%, [M + H]+). 6d MS (ESI+) m/z 445.5 (100%, [M + H]+). 6e MS (ESI+) m/z 459.4 (100%, [M + H]+). 6f MS (ESI+) m/z 473.4 (100%, [M + H]+). 6g MS (ESI+) m/z 487.6 (100%, [M + H]+). 6h MS (ESI+) m/z 499.6 (100%, [M + H]+). 6m MS (ESI+) m/z 573.6 (100%, [M + H]+). 8b MS (ESI+) m/z 400.6 (100%, [M + H]+). 8c MS (ESI+) m/z 429.4 (100%, [M + H]+). 8d MS (ESI+) m/z 443.5 (100%, [M + H]+). 8e MS (ESI+) m/z 457.5 (100%, [M + H]+).
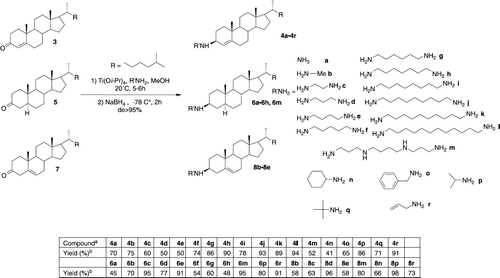
Scheme 2 Titanium(IV) Reductive Amination Reaction of 4-Cholesten-3 one 3 cholestan-3-one 5 and 5-Cholesten-3-one 7 using various amines aReactions performed at 20°C for 12 h in MeOH on a 0.78 mmol scale of ketone 5 or 7 in the presence of Ti(Oi-Pr)4 (1.03 mmol) and the desired amine (2.34 mmol). bIsolated yields.
Determination of minimal inhibitory concentrations
Antimicrobial activity of the compounds was studied by determination of minimal inhibitory concentrations (MIC) according to the NCCLS guidelines using the microbroth dilution methods.[Citation26] The cells were grown overnight at 28°C (S. cerevisiae ATCC 28383) or 37°C (E. coli ATCC 10536, S. aureus ATCC 6538, E. faecalis CIP 103015, C. albicans ATCC 90029, C. tropicalis CIP 2031) in YPD broth for S. cerevisiae and Sabouraud broth for C. albicans and C. tropicalis, LB broth for E. coli and S. aureus or BHI broth for S. faecalis. 8 μL of a compound solution of 5 mg/mL were serially diluted by factor two with the corresponding broth. Concerning E. hirae CIP 5855 all the tests were realized according AFNOR recommandations using Mueller-Hinton broth. Econazole and streptomycin were used as substrate reference for all fungi and bacteria, respectively. The bacteria strains were grown on trypticase soy agar (Becton Dickinson) at 37°C for 24 h and the yeast on Sabouraud agar for 48 h. Inocula were prepared in TCE (tryptone 0.1%, NaCl 8,5 %, wt/vol) by ajusting the turbidity at 623 nm to obtain 1–3 105CFU/mL.
Broth microdilution method was used to determine the MIC and was performed in sterile 96-well microplates. Each compounds (5 mg/mL in methanol) was transferred to each microplate well, in order to obtain a two-fold serial dilution in 100μL of broth and 100 μL of inocula containing 2–6 105 CFU of each bacteria and yeast were added to each well. A number of wells were reserved for positive controls, inoculum viability and solvent effect. After 24 or 48 h incubation, growth was assayed by absorbance measurement at 623 nm with an ELX 808 IU (Biotek Instruments). MIC was defined for each agent from duplicate observations as the lowest concentration of compound allowing no visible growth.
Results and Discussion
Chemical design of 3-amino and polyaminosterol derivatives
The chemical stuctures of the 36 new aminosterol derivatives are presented in Scheme . All these squalamine mimics in which various polyamino groups are attached at position C-3 to a variety of steroid molecules were produced in high chemical yield with readily available starting materials and a simple one step procedure involving a stereoselective titanium reductive amination procedure.
Antimicrobial activities of 3-amino and polyaminosterol derivatives
Quantitative MIC were determined by means of the broth dilution method. summarizes the antifungal and antibacterial activities of the synthesized amino and polyaminosterol derivatives. In this context, several squalamine mimics showed significant activities against fungi and Gram-positive bacteria comparable to those obtained with an authentic sample of squalamine.
Table I. Antimicrobial Activities of Aminosterol Derivatives 4, 6, 8.
In this study, among the 37 molecules tested against the 3 yeast strains, only derivatives 4b, 4e, 4n and 6b were active simultaneously on S. cerevisiae, C. albicans and C. tropicalis. The most sensitive strain was S. cerevisiae presenting MIC range of 6.25–25 μM for 10 compounds. In this area, compounds 4r and 8b (MIC = 6.25 μM) possess the most-potent antifungal activities against this latter strain. C. albicans was the less sensitive yeast since only 4 compounds showed MIC ≤ 25 μM (4b, 4e, 6b, 8n). Additionally, 5 products (4b, 4e, 4j, 4n and 8n) present MIC ≤ 12.5 μM against C. tropicalis, this latter strain seeming to be most sensitive towards these derivatives.
Finally, best results were encountered with derivatives 4b, 4e, 4n leading to MIC varying from 6.25 to 25 μM on all the considered strains.
This series were tested on bacteria Gram + and Gram-. Activity on bacteria Gram negative were weak. Only 3 compounds (8c, 8d, 8e) versus 37 exhibit antibacterial activities against E. coli (MIC ≤ 12.5 μM). These results demonstrate that nature of the amino group attached to the sterol moiety plays an important role on the potential activities of our products. Nevertheless, to date, no pertinent explanation can be demonstrated but since membrane lysis could occur through pore formation, this fact suggests that the binding sites of these cationic steroids are lipopolysaccharides constituents of Gram-negative membrane bacteria.
Moreover, more interesting activities were observed against Gram-positive bacteria. E. hirae was more sensitive bacteria with respect to E. faecalis. Almost of all these compounds possess important antibacterial activity against this strain with a best value for 4i (MIC = 3.12 μM) since only 7 compounds were inactive at concentrations below 6.25 μM. For S. aureus, 19 products expressed antibacterial activities with MIC ≤ 50 μM, the most active being 4b, 4n, 4p, 4r (MIC = 3.12–6.25 μM).
Although it is difficult to establish pertinent structure-activity relationships, it can be reasonably envisaged that the target sites of such compounds, having numerous positive charges, are lipoteichoic or teichoic acids which are negatively charged and are important constituant of the cell wall structure of Gram positive bacteria.
Compound 4e bearing a putrescine moiety presents the best antimicrobial activities of all the tested strains and at present time, we investigate the possibility for this product to be an inhibitor of spermidine synthase. Furthermore, results obtained from trodusquemine analogues 4m clearly suggest that the antimicrobial activities measured are very sensitive to the structure of the sterol derivative involved. On the other hand, the saturated derivative 6m differing only from trodusquemine 2 by the absence of hydroxy and sulfate groups led to similar antimicrobial activities against yeasts and Gram positive bacteria whereas no antibacterial activity against E. coli was noticed suggesting the importance of these two functional groups in the mechanism of action of trodusquemine. Finally, derivative 4n bearing a cyclohexylamine moiety led to moderate to excellent results against fungi and Gram positive bacteria even if to date no pertinent explanations can be rationalized.
Conclusion
In conclusion, synthesis of a series of 3-amino- and polyaminosterol analogues of squalamine and trodusquemine has been realized. Numerous of these synthetic derivatives present interesting antimicrobial activities on various different fungal and bacterial strains. Extension of such a methodology for the synthesis of new amino-and polyaminosterol compounds in order to improve the biological activity of such derivatives is in progress and will be reported in due course.
References
- HG Boman. (1991). Antibacterial peptides: Key components needed in immunity. Cell 65:205–207.
- J Cho, and Y Kim. (2002). Sharks: A Potential Source of Antiangiogenic Factors and Tumor Treatments. Marine Biotechnol 4:521–525.
- R Stone. (1993). Deja vu guides the way to new antimicrobial steroid. Science 259:1125.
- M Zasloff. (1992). Curr Opin Immunol 4:3–7.
- M Zasloff. (1994). Antibacterial molecules from frogs, sharks and man. Phylogenet Perspect Immun Insect Host Def 31–41.
- SL Wehrli, KS Moore, H Roder, S Durell, and M Zasloff. (1993). Structure of the novel steroidal antibiotic squalamine determined by two-dimensional NMR spectroscopy. Steroids 58:370–378.
- PB Savage. (2002). Cationic steroid antibiotics. Curr Med Chem-Anti-Infective Agents 1:293–304.
- PB Savage. (2002). Design, synthesis and characterization of cationic peptide and steroid antibiotics. Eur J Org Chem 759–768.
- KS Moore, S Wehrli, H Roder, M Rogers, JN ForrestJr, D McCrimmon, and M Zasloff. (1993). Squalamine: An aminosterol antibiotic from the shark. Proc Natl Acad Sci USA 90:1354–1358.
- MN Rao, AE Shinnar, LA Noecker, TL Chao, B Feibush, B Snyder, I Sharkansky, A Sarkahian, X Zhang, SR Jones, WA Kinney, and M Zasloff. (2000). Aminosterols from the dogfish shark Squalus acanthias. J Nat Prod 63:631–635.
- JM Brunel, and Y Letourneux. (2003). Recent advances in the synthesis of spermine and spermidine analogs of the shark aminosterol squalamine. Eur J Org Chem 3897–3907.
- JM Brunel, C Salmi, C Loncle, N Vidal, and Y Letourneux. (2005). Squalamine: A polyvalent drug of the future. Curr Cancer Drug Targets 5:267–272.
- K Hamana, and S Matsuzaki. (1992). Polyamines as a chemotaxonomic marker in bacterial systematics. Crit Rev Microbiol 18:261–283.
- HM Wallace. (1998). Polyamines: Specific metabolic regulators or multifunctional polycations?. Biochem Soc Trans 26:569–571.
- HM Wallace. (2000). The physiological role of the polyamines. Eur J Clin Inv 30:1–3.
- HM Wallace. (2003). Polyamines and their role in human disease - an introduction. Biochem Soc Trans 31:354–355.
- FR Saunders, and HM Wallace. (2007). Polyamine metabolism and cancer prevention. Biochem Soc Trans 35:364–368.
- K Yasuda, C Ohmizo, and T Katsu. (2004). Mode of action of novel polyamines increasing the permeability of bacterial outer membrane. Int J of Antimicrob Agents 24:67–71.
- HS Kim, BS Choi, KC Kwon, SO Lee, HJ Kwak, and CH Lee. (2000). Synthesis and antimicrobial activity of squalamine analogue. Bioorg Med Chem 8:2059–2065.
- K Kikuchi, EM Bernard, A Sadownik, SL Regen, and D Armstrong. (1997). Antimicrobial activities of squalamine mimics. Antimicrob Agents Chemother 41:1433–1438.
- SR Jones, WA Kinney, X Zhang, LM Jones, and BS Selinsky. (1996). The synthesis and characterization of analogs of the antimicrobial compound squalamine: 6β-hydroxy-3-aminosterols synthesized from hyodeoxycholic acid. Steroids 61:565–571.
- A Sadownik, G Deng, V Janout, SL Regen, EM Bernard, K Kikuchi, and D Armstrong. (1995). Rapid Construction of a Squalamine Mimic. J Am Chem Soc 117:6138–6139.
- S Khabnadideh, CL Tan, SL Croft, H Kendrick, V Yardley, and IH Gilbert. (2000). Squalamine analogs as potential anti-trypanosomal and anti-leishmanial compounds. Bioorg Med Chem Lett 10:1237–1239.
- C Salmi, Y Letourneux, and JM Brunel. (2006). Efficient synthesis of various secondary amines through a titanium(IV) isopropoxide-mediated reductive amination of ketones. Lett Org Chem 3:396–401.
- C Salmi, Y Letourneux, and JM Brunel. (2006). Efficient diastereoselective titanium(IV) reductive amination of ketones. Lett Org Chem 3:384–389.
- National commitee for clinical laboratory standards. 1992. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A2. National commitee for clinical laboratory standards, Villanova, Pa..