Abstract
Flavonoids are an important group of natural compounds that can interfere with the activity of some enzymes. In this study, effects of various flavonoids on aldehyde oxidase (AO) activity were evaluated in vitro. AO was partially purified from guinea pig liver. The effects of 12 flavonoids from three subclasses of flavon-3-ol, flavan-3-ol and flavanone on the oxidation of vanillin and phenanthridine as substrates of AO and xanthine as a substrate of xanthine oxidase (XO) were investigated spectrophotometrically. Among the 12 flavonoids, myricetin and quercetin were the most potent inhibitors of both AO and XO. In general, the oxidation of vanillin was more inhibited by flavonoids than that of phenanthridine. Almost all of the flavonoids inhibited AO activity more potently than XO, which was more evident with non-planner flavanols. A planner structure seems to be essential for a potent inhibitory effect and any substitution by sugar moieties reduces the inhibitory effects. This study could provide a new insight into AO natural inhibitors with potential to lead to some food-drug interactions.
Introduction
Drug-drug interactions are of great concern that may lead to increased side effects and decreased efficacy of the drugs. The extensive parts of drug interactions arise via interaction of compounds with drug-metabolizing enzymes. Aldehyde oxidase (AO, aldehyde: O2 oxidoreductase EC 1.2.3.1) is a molybdenum cofactor-containing cytosolic enzyme which is widely distributed throughout the animal kingdom [Citation1–3]. The enzyme is predominantly active in liver and other tissues of mammalian species and involved in the metabolism of extensive range of aldehydes and nitrogen-containing compounds with physiological, pharmacological, and toxicological relevance such as pyridoxal [Citation4], retinal [Citation5], allopurinol [Citation6], monoamine neurotransmitters [Citation7], famciclovir [Citation8], methotrexate [Citation9], quinine [Citation10], carbazeran [Citation11], zaleplon [Citation12], N-methylnicotinamide [Citation13] and nicotine [Citation14]. In addition, it participates significantly in the metabolism of intermediary aldehyde metabolites of alcohol, citalopram [Citation15] and tamoxifen [Citation16]. There are also a large number of reports indicating that AO is capable of catalyzing the reduction of a broad type of compounds in the presence of appropriate electron donors [Citation17–20]. This broad range of substrates indicates that AO has a relatively active binding site. However, in spite of this broad substrate activity, this enzyme has not received enough attention as a target of drug-drug interactions [Citation21].
AO has many physicochemical properties similar to those of other molybdenum containing enzyme, xanthine oxidoreductase [XOR, xanthine: O2 oxidoreductase EC 1.2.3.2]. Like AO, XOR can oxidize both aldehydes and N-heterocyclic compounds, however the former enzyme has a broader substrate specificity, compared with XOR [Citation1,Citation21,Citation22]. Many natural components such as flavonoids have been shown to interfere with XOR-catalyzed reactions. Flavonoids belong to polyphenolic compounds ubiquitously found in nature including fruits, vegetables, remedies, grains, bark, roots, stems, flowers, tea and wine [Citation23]. The pharmacological activities of flavonoids are mainly attributed to their potentials for inhibition of certain enzymes and their antioxidant activity [Citation24]. The flavonoid family frequently addressed as a potent candidate for inhibition of xanthine oxidase (XO), and their structural features were revealed to induce corresponding role [Citation24–29]. Our recent published results have shown that the extract of R. graveolens L. which is rich of flavonoids, potently inhibits AO [Citation32]. Although particular efforts are focused on the inhibitory effects of flavonoids on XO activity [Citation29,Citation30], to date, the possible interfering effects of flavonoids on AO activity has not received any attention. Therefore, in the present study, the possible inhibitory effects of some flavonoids from three subclasses of flavon-3-ol, flavan-3-ol and flavanone () on guinea pig liver AO would be documented. Guinea pig liver was used as the source of enzyme because of its similarity to the human hepatic AO [Citation2]. The data could be of value in identification of some food-drug interactions that could be observed between flavonoids-rich foods and the drugs metabolized by this enzyme. The results would be also useful in the development of structure-activity relationships for AO inhibition.
Material and methods
Reagents
Myricetin, morin, hyperoside, rutin, quercetin, catechin, epicatechin, naringenin, naringin, hesperetin, hesperidin, taxifolin, allopurinol, menadione, phenanthridine, xanthine and buttermilk bovine xanthine oxidase (Grade I, from buttermilk, 0.75 units/mg protein) were purchased from Sigma-Aldrich (Poole, Dorset, England). Vanillin was supplied by Merck (KgaA, Darmstadt, Germany). Bicinchoninic acid (BSA®) protein assay kit was obtained from Pierce Chemicals (Rockford, U.S.A).
Enzyme preparation
Partially purified AO was prepared from mature male Dunkin-Hartley guinea pigs (400–600 g, Tabriz University of Medical Sciences, Tabriz, Iran) from the liver homogenates by heat treatment and ammonium sulfate precipitation as described before [Citation32,Citation33].
Enzyme assays
Guinea pig hepatic AO activity was assayed spectrophotometrically using phenanthridine (an N-heterocyclic compound) and vanillin (an aldehyde) as substrates at 322 and 310 nm, respectively. All spectrophotometric determinations were carried out at 37°C using a Shimadzu 2550 UV/VIS spectrophotometer which was controlled by the Shimadzu UV Probe personal software package. The instrument was connected to a Shimadzu cell temperature control unit. Each substrate was separately incubated with the enzyme fraction in Sorenson's phosphate buffer pH 7.0 containing 0.1 mM of EDTA at 37°C at final concentration of 50 μM. The reaction started by addition of the enzyme fraction and monitored up to two minutes. Xanthine oxidase activity was measured by monitoring of uric acid production at 295 nm. Xanthine (50 μM), as specific substrate for XO, was incubated with the enzyme fraction in Sorenson's phosphate buffer (67 mM, pH 7.0) containing 0.1 mM EDTA and the initial oxidation rates were measured up to 3 minutes.
The reactions were also measured in the presence of flavonoids (1–100 μM) and the results were compared with the inhibitory effects of 1–100 μM menadione (an AO specific inhibitor) and 1–100 μM allopurinol (a potent inhibitor of xanthine oxidase). Prior to any assay of initial reaction rates, all solutions, apart from the enzyme which was stored on ice bath before addition to the incubation mixture, were equilibrated at 37°C. Any other possible enzymatic and non-enzymatic interactions of the flavonoids in the absence of AO substrates with the components of the incubation solution were tested prior to the measurement of the substrate oxidations.
Determination of kinetic constants
The values for Michaealis-Menton constant (Km) and maximum velocity (Vmax) were determined from the Lineweaver-Burk plots obtained from 1/v (nM/min/mg protein) vs. 1/[S] (μM). The initial oxidation rates were performed for at least at five different substrate concentrations. The line of the best fit across the points was estimated using simple linear regression by the least square method.
Inhibition studies
The effect of inhibitors on the initial oxidation rate of substrates was also investigated spectrophotometrically. Flavonoid inhibition studies were carried out using at least two concentrations of each inhibitor. The inhibition constant (Ki) was determined using secondary plot (slopes and intercepts from the initial Lineweaver–Burk plot vs. inhibitor concentrations). In the case of mixed inhibition where inhibitor can bind to the free enzyme (EI) and to the enzyme–substrate complex (ESI), two inhibitor constants were defined as Ki (the dissociation constant of the enzyme–inhibitor complex), and KI (the dissociation constant of the enzyme–substrate–inhibitor complex). If Ki < KI, the inhibition was considered as a competitive-noncompetitive type of the inhibition; if Ki > KI, the inhibition was termed as uncompetitive-noncompetitive type. In the case of Ki = KI, the inhibition was considered as a pure noncompetitive inhibition.
The kinetic mechanism of flavonoids, which exhibited lower degrees of inhibition, were not investigated further, because of their limited potencies.
The IC50 (The inhibitor concentration that causes 50% inhibition) values were obtained from the plot of the log of more than six concentrations of the tested compound vs. the percent inhibition of the enzyme activities.
Protein content estimation
Protein concentrations of partially purified enzyme fractions were determined spectrophotometrically using a Pierce BCA Protein assay kit with bovine serum albumin as a protein standard according to the method of Smith et al. [Citation33].
Results
Inhibition of AO and XO by flavonoids
As a preliminary step, certain subsets of flavonoids–flavonols, flavanoles and flavanones () were examined for their potency to inhibit AO activity. In the degree of inhibition on the activity of AO and XO was summarized. All comparisons here are considered at 10 μM concentration of inhibitors. AO-linked activities were all inhibited notably by menadione at 10 μM (range, 72.9 to 95.5%). Allopurinol (oxypurinol) was a potent inhibitor of XO, at higher concentration caused 7.1 and 6.2% inhibition on the vanillin and phenanthridine oxidations, respectively. Among selected flavonoids, quercetin (73.4–92.7%) and myricetin (72.9–90.5%) were evidently involved as the most potent inhibitors of enzyme-catalyzed oxidations, which were comparable to that of menadione as a positive control (89.6–96.3%). Rutin, a 3-rutinosyl derivative of quercetin, showed far less inhibitory effect than quercetin on the oxidation of vanillin and phenanthridine. Hyperoside, a 3-galactosiyl derivative of quercetin, showed much less inhibitory activity as rutin. Morin induced moderate inhibition on AO activity, while comparing with quercetin and myricetin. However, morin affected the oxidation of vanillin (76.2%) more effective than phenanthridine (25.8%). Catechin and epicatechin exerted rather high inhibition on the oxidation of vanillin and moderate inhibition toward the oxidation of phenanthridine. In the case of AO, aglycons and methoxy derivatives of flavanones were found to be fairly potent inhibitors of the enzyme, whereas, naringenin and hesperetin acted as moderately potent inhibitors of AO. In contrast, the substitutions of 7-hydroxyl moiety with neohespridose in naringin induced hallmark depression of the naringenin capability toward inhibition of AO- and XO-catalyzed reactions. Hesperidin, 7-derivative of hesperetin, was the weakest inhibitor. In overall, all tested flavonoids virtually exhibited more potent inhibition against the oxidation of vanillin rather than phenanthridine.
Table I. The effects of some flavonoids on the oxidation of vanillin and phenanthridine catalyzed by guinea pig liver AO and xanthine oxidation catalyzed by guinea pig liver XO.
The IC50 values for inhibitory effect of flavonoids on AO and XO activity
As shown in , some flavonoids exerted their inhibitory effects in a dose-dependent manner. Based on the obtained IC50 values, myricetin (range, 0.38 to 0.71 μM), quercetin (range, 0.41 to 2.11 μM) and menadione (range, 0.38 to 1.9 μM) were the most potent inhibitors. They exhibited more selective inhibition on AO-catalyzed oxidations toward vanillin than phenanthridine. Naringin and hesperidin displayed negligible inhibitions among tested flavonoids. Other flavanones and flavanols caused moderately high inhibition on the AO activity. The IC50 values for the inhibition of vanillin and phenanthridine oxidations by catechin were 2.5 and 6.1 μM, respectively. The corresponding values obtained for epicatechin were 5.7 μM and 6.1 μM, respectively. In contrast, XO activity was inhibited by approximately 10-fold higher concentration of catechin (IC50 = 70.9 μM) and epicatechin (IC50 = 59.2 μM). However, morin, taxifolin and quercetin were found to be relatively more potent inhibitors of XO than AO.
Table II. IC50 values for the inhibition of guinea pig liver AO and XO by various flavonoids, menadione and allopurinol.
Inhibition kinetic parameters of flavonoids on the oxidation of vanillin and phenanthridine
For further exploring the inhibitory characteristics of flavonoids, the kinetic assay was performed using Lineweaver-Burk double reciprocal plots. The kinetic parameters have been listed in . AO was inhibited by flavonols in a mixed-inhibition manner. In particular, KI values of myricetin and quercetin for the oxidation of vanillin were lower than the corresponding Ki values indicating a higher affinity of inhibitors to EI complex; whereas for the oxidation of phenanthridine, the Ki values were lower than the corresponding KI values indicating a mixed inhibition with a marked competitive affinity (ESI). Although the presence of the attached sugar moiety to quercetin resulted in a significant loss of inhibitory activity with both substrates, hyperoside and rutin exhibited higher tendency to form enzyme-substrate-inhibitor complex (ESI). Two inhibition constants were obtained for the inhibition of either vanillin or phenanthridine by morin. However, there were no marked differences between the values. Therefore, it would be difficult to consider the inhibitions as mixed one. Regarding the type of substrate, vanillin was inhibited more potently than phenanthridine by morin (). The aldehyde oxidation was inhibited in a pure non-competitive manner by either catechin or epicatechin; however, these two flavonoids inhibited AO-catalyzed oxidation of phenanthridine competitively.
Table III. Kinetic characteristics of the inhibitory activity of flavonoids on the oxidation of vanillin and phenanthridine by guinea pig liver AO.
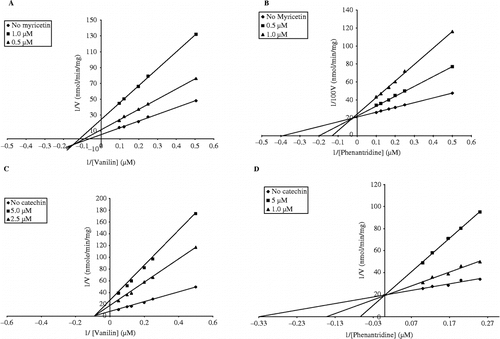
The characterized potent inhibitors of flavanones were of interest and selected for further kinetic analysis. As a whole, they acted in a non-competitive mode toward the oxidation of vanillin with Ki values approximately ranged 2.0–3.4 μM, whereas, mixed-mode of inhibition was notable for the oxidation of phenanthridine. In , typical Lineweaver-Burk plots for inhibitory activity of myricetin and catechin on the oxidation of vanillin and phenanthridine catalyzed by guinea pig liver AO have been illustrated.
Figure 2. Typical Lineweaver-Burk plots for inhibitory activity of myricetin and catechin on the oxidation of vanillin (A and C, respectively) and phenanthridine (B and D, respectively) catalyzed by guinea pig liver AO. The enzyme assays were performed as described in materials and methods. The data represent the average of 4–8 experiments.
Discussion
Aldehyde oxidase is a cytosolic enzyme that is actively involved in the metabolism of xenobiotics including some important drugs which possess aldehyde and nitrogenous heterocyclic elements [Citation1–4]. There are sufficient reports indicating that flavonoids, as important natural compounds, may act as potent inhibitors of XO [Citation23–31]. Because of structural-functional similarities between XO and AO [Citation21,Citation30], flavonoids could have potential to interfere with AO activity. Our recent published data showed that R graveolens L. extract and its major flavonoid, quercetin, inhibit AO activity [Citation32]. To date, there is no any comprehensive report indicating the probable interaction between flavonoids and AO. In this study, myricetin and quercetin were found as the most potent inhibitors of AO and XO. The inhibitory effects were comparable to those obtained for allopurinol (the potent inhibitor of XO) and menadione (the specific and potent AO inhibitor). Myricetin (3′,4′,5′-OH) with additional hydroxyl group at C5′ (vs. to quercetin with 3′,4′-OH) showed slightly higher potency of inhibition toward AO. Van Hoorn et al. [Citation24] suggested that hydroxyl moieties at C3′ and C4′ are important for inducing a potent inhibition against XO activity. These inhibitory effects of quercetin and myricetin on aldehyde oxidase activity can lead to some food-drug interactions. According to Aziz et al., after consumption of 300 g samples of lightly fried onions, the mean peak plasma level of quercetin can reach to 7.0 μM [Citation34]. In other study [Citation35], the peak plasma concentration of quercetin following ingestion of 225 g fried onions was found to be 248.4 ng/ml (0.83 μM). More recently, Zhang et al. [Citation36] have shown that myricetin maximum plasma concentration in rat plasma after orally administrating decoction of Rattan Tea is 0.47 μg/ml (1.48 μM). Bearing in mind that both flavonoids can cause ≥ 75% inhibition on aldehyde oxidase activity at 1 μM (), it would be more likely that some drug interactions occur following consumption of foods or beverages which are rich in these flavonoids. Compared with quercetin, morin showed relatively less inhibition on both AO and XO. The lack of 3′-hydroxyl moiety in morin and the additional 2′-hydroxyl moiety substitution resulted in reduction in the inhibition on AO. It has been pronounced that hydroxyl group at C2′ position has the greatest inhibitory effects on XO activity [Citation24], which is consistent with our report for XO.
Substitution of hydroxyl moiety at C3 of quercetin (rutin and hyperoside) caused marked loss of inhibition on AO. It has been documented that the substitution of hydroxyl group at C3 of C ring by sugar molecules can reduce XO [Citation24,Citation25], and AO activities [Citation32]. Similarly, identical structural characteristics have been reported for structure-activity relationship of steroids and AO. It has been shown that substitution of C3 of A ring in β-steradiol reduces the inhibitory effect of the steroid on AO activity [Citation37,Citation38]. According to Lin et al. [Citation26], the reduction in the XO activity could be resulted from the destabilization of the polar hydroxyl stretching into the hydrophobic region of active site of the enzyme and lowering binding affinity.
Interestingly, in general, the examined flavanoids caused more inhibition on the oxidation of vanillin, as an aldehyde substrate of AO, than phenanthridine, as a N-heterocyclic compound. To our knowledge, this finding is the first evidence indicating that the activity of AO towards an aldehyde substrate compared to an N-heterocyclic one is inhibited differently by a structurally related group of compounds. Particularly, morin acted as the most selective inhibitor toward the oxidation of vanillin, while it was compared with phenanthridine. The difference between the inhibitory effects of flavonoids on the AO towards vanillin and phenanthridine was also confirmed by the kinetic studies ().
Another interesting result obtained in this study was that the tested flavon-3-ols had almost similar inhibitory effects on AO and XO activities, whereas two other subclasses (the flavan-3-ols and flavanones) caused more inhibition on AO, in particular vanillin oxidation, than XO. It appears that the flavonoids with a non-planner structure cause less inhibition on XO activity than those have a planner structure. These results are consistent with those reports postulating that a planner structure of flavonols is essential for inhibitory potentials against XO [Citation24,Citation26,Citation29]. However, this effect was not significant with AO ( and ).
Conclusion
According to the results obtained in this study, flavonoids as important natural compounds could inhibit AO activity; some of these such as quercetin and myricetin were found to be as potent as the putative inhibitor of AO, menadione. As flavonoids ubiquitously exist in the nature, particularly in foods, these findings could be important from a drug-food interaction point of view which is warranted for further investigations. It would be of value to bear in mind that unlike other drug-metabolizing enzymes such as cytochrome P450, there is paucity evidence on the interaction of natural compounds with AO-catalyzed reactions. This study also provided some evidence indicating a difference between the inhibitory effects of a group of compounds on AO activity towards aldehyde and N-heterocyclic substrates. It was also revealed that some differences between AO and XO exist in terms of their interaction with flavonoids.
Acknowledgements
The authors would like to thank the Research Affairs Office of Tabriz University of Medical Sciences for its financial support.
References
- C Beedham. Molybdenum hydroxylases. In: C Ioannides, editor. Enzyme systems that metabolites drugs and other xenobiotics. New York: John Wiley;. (2002). p 147–148.
- C Beedham. Molybdenum hydroxylases: Biological distribution and substrate-inhibitor specificity. In: GP Ellis, and GP West, editors. Progress in medicinal chemistry. Amsterdam: Elsevier Science Publishers (Biomedical Division); (1987). p 58–127.
- C Beedham. Molybdenum hydroxylase. In: JW Gorrod, editor. Metabolism of xenobiotics. London and New York: Taylor & Francis; (1998). p 51–58.
- M Stanulovic, and S Chaykin. (1971). Aldehyde oxidase: Catalysis of the oxidation of N1-methylnicotinamide and pyridoxal. Arch Biochem Biophys 145:27–34.
- W Ambroziak, G Izaguirre, and R Pietruszko. (1999). Metabolism of retinaldehyde and other aldehydes in soluble extracts of human liver and kidney. J Biol Chem 274:33366–33373.
- Y Moriwaki, T Yamamoto, J Yamakita, S Takahashi, Z Tsutsumi, and K Higashino. (1998). Zonal distribution of allopurinol-oxidizing enzymes in rat liver. Adv Exp Med Biol 431:47–50.
- G Panoutsopoulos. (2005). Metabolism of homovanillamine to homovanillic acid in guinea pig liver slices. Cell Physiol Biochem 15:225–232.
- MR Rashidi, JA Smith, SE Clarke, and C Beedham. (1997). In vitro oxidation of famciclovir and 6-deoxypenciclovir by aldehyde oxidase from human, guinea pig, rabbit, and rat liver. Drug Metab Dispos 25:805–813.
- CG Jordan, MR Rashidi, H Laljee, SE Clarke, JE Brown, and C Beedham. (1999). Aldehyde oxidase-catalysed oxidation of methotrexate in the liver of guinea-pig, rabbit and man. J Pharm Pharmacol 51:411–418.
- C Beedham, Y al-Tayib, and JA Smith. (1992). Role of guinea pig and rabbit hepatic aldehyde oxidase in oxidative in vitro metabolism of cinchona antimalarials. Drug Metab Dispos 20:889–895.
- DJ Critchley, DJ Rance, and C Beedham. (1994). Biotransformation of carbazeran in guinea pig: Effect of hydralazine pretreatment. Xenobiotica 24:37–47.
- BG Lake, SE Ball, J Kao, AB Renwick, RJ Price, and JA Scatina. (2002). Metabolism of zaleplon by human liver: Evidence for involvement of aldehyde oxidase. Xenobiotica 32:835–847.
- K Sugihara, Y Tayama, K Shimomiya, D Yoshimoto, S Ohta, and S Kitamura. (2006). Estimation of aldehyde oxidase activity in vivo from conversion ratio of N1-methylnicotinamide to pyridines, and interspecies variation of the enzyme activity in rats. Drug Metab Dispos 34:208–212.
- S Brandange, and L Lindblom. (1979). The enzyme “aldehyde oxidase” is an iminium oxidase. Reaction with nicotine delta 1′(5′) iminium ion. Biochem Biophys Res Commun 91:991–996.
- B Rochat, M Kosel, G Boss, B Testa, M Gillet, and P Baumann. (1998). Stereoselective biotransformation of the selective serotonin reuptake inhibitor citalopram and its demethylated metabolites by monoamine oxidases in human liver. Biochem Pharmacol 56:15–23.
- PC Ruenitz, and X Bai. (1995). Acidic metabolites of tamoxifen. Aspects of formation and fate in the female rat. Drug Metab Dispos 23:993–998.
- S Kitamura, KNK Ohashi, K Sugihara, R Hosokawa, Y Akagawa, and S Ohta. (2001). Extremely high drug-reductase activity based on aldehyde oxidase in monkey liver. Biol Pharm Bull 24:856–859.
- SC Lee, and AG Renwick. (1995). Sulphoxide reduction by rat and rabbit tissues in vitro. Biochem Pharmacol 49:1557–1565.
- K Tatsumi, H Yamada, and S Kitamura. (1983). Evidence for involvement of liver aldehyde oxidase in reduction of nitrosamines to the corresponding hydrazine. Chem Pharm Bull 31:764–767.
- K Tatsumi, S Kitamura, and H Yamada. (1983). Sulfoxide reductase activity of liver aldehyde oxidase. Biochem Biophys Acta 747:86–92.
- RS Obach. (2004). Potent inhibition of human liver aldehyde oxidase by raloxifene. Drug Metab Dispos 32:89–97.
- R Hille. (2005). Molybdenum-containing hydroxylases. Arch Biochem Biophys 433:107–116.
- RJ Nijveldt, E van Nood, DEC van Hoorn, PG Boelens, K van Norren, and PAM van Leeuwen. (2001). Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425.
- DE Van Hoorn, RJ Nijveldt, PA Van Leeuwen, Z Hofman, L M'Rabet, DB De Bont, and et al (2002). Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur J Pharmacol 451:111–118.
- J Aucamp, A Gaspar, Y Hara, and Z Apostolides. (1997). Inhibition of xanthine oxidase by catechins from tea (Camellia sinensis). Anticancer Res 17:4381–4385.
- CM Lin, CS Chen, CT Chen, YC Liang, and JK Lin. (2002). Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem Biophys Res Commun 294:167–172.
- J Hanaee, MR Rashidi, A Delazar, and S Pirouzpanah. (2004). Onion, a potent inhibitor of xanthine oxidase. Iran J Pharm Res 4:243–247.
- A Nagao, M Seki, and H Kobayashi. (1999). Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem 63:1787–1790.
- N Cotelle. (2001). Role of flavonoids in oxidative stress. Curr Top Med Chem 1:569–590.
- S Kitamura, K Sugihara, and S Ohta. (2006). Drug-metabolizing ability of molybdenum hydroxylases. Drug Metab Pharmacokinet 21:83–98.
- Z Yu, WP Fong, and CH Cheng. (2006). The dual actions of morin (3,5,7,2′,4′-pentahydroxyflavone) as a hypouricemic agent: Uricosuric effect and xanthine oxidase inhibitory activity. J Pharmacol Exp Ther 316:169–175.
- S Pirouzpanah, MR Rashidi, A Delazar, SV Razavieh, and A Hamidi. (2006). Inhibitory effects of Ruta graveolens L. Extract on guinea pig liver aldehyde oxidase. Chem Pharm Bull 54:9–13.
- PK Smith, RI Krohn, GT Hermanson, AK Mallia, FH Gartner, MD Provenzano, EK Fujimoto, NM Goeke, BJ Olson, and DC Klenk. (1985). Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85.
- AA Aziz, CA Edwards, MEJ Lean, and A Crozier. (1998). Absorption and excretion of conjugated flavonols, including quercetin-4′-O-β-glucoside and isorhamnetin-4′-O-β-glucoside by human volunteers after the consumption of onions. Free Rad Res 29:257–269.
- GT McAnlis, J McEneny, J Pearce, and IS Young. (1999). Absorption and antioxidant effects of quercetin from onions, in man. Eur J Clin Nutr 53:92–96.
- Y Zhang, Q Zhang, L Li, B Wang, Y Zhao, and D Guo. (2007). Simultaneous determination and pharmacokinetic studies of dihydromyricetin and myricetin in rat plasma by HPLC-DAD after oral administration of Ampelopsis grossedentata decoction. J Chromatogr B 860:4–9
- K Huh, US Shin, JW Choi, and SI Lee. (1994). Effect of sex hormones on lipid peroxidation in rat liver. Arch Pharmacol Res 17:109–114.
- DU Lee, US Shin, and K Huh. (1998). Structure-activity relationships of gagaminine and its derivatives on the inhibition of hepatic aldehyde oxidase activity and lipid peroxidation. Arch Pharmacol Res 21:273–277.