Abstract
The synthesis, characterization and pharmacological activities of a new series of (6-difluorobenzoyl)-5-methyl-3-benzoylmethyl-2(3H)-benzoxazolone and 5-methyl-3-(2-hydroxyl-2-phenylethyl)-2(3H)-benzoxazolone are described. Antiinflammatory activity was investigated by the carrageenin-induced paw oedema test and analgesic activity by acetic acid writhing and hot plate tests in mice. Among the synthesized compounds, compound 3e 6-(2,5-difluorobenzoyl)-3-(4-bromobenzoylmethyl-2(3H)-benzoxazolone was found to be the most promising compound for analgesic activity. Reduced compounds (4a–4d) displayed considerable anti-inflammatory activity compared to the other derivatives.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a main-stay in the treatment of inflammation and they owe their therapeutic and side effects in large part to the inhibition of cyclooxygenase (COX). The separation of the therapeutic effects from the side effects has been a major challenge in the design and synthesis of these drugs. The discovery of a second isoform of cyclooxygenase, namely COX-2, has opened a new line of research based on the assumption that pathological prostaglandins are produced by the inducible isoform COX-2 while physiological prostaglandins are produced by the constitutive isoform COX-1 [Citation1]. On this premise several new inhibitors have been developed and some are now commercially available [Citation2–3]. However, an increased risk of myocardial infarction and cardiovascular thrombotic events associated with the use of some selective COX-2 inhibitors has been observed [Citation4]. These adverse cardiovascular effects, which are attributed to a decreased level of the vasodilatory PGI2 and an increased level of the potent platelet aggregator TxA2, were primarily responsible for the recent withdrawal of rofecoxib (Vioxx®) and valdecoxib (Bextra®) from the market [Citation5]. On our going medicinal chemistry research programme, we have seen that 2(3H)-benzoxazolones may be a building block in the drug structures and these structures bearing 2(3H)-benzoxazolones exhibit wide range of biological activities such as analgesics-antiinflammmatory [Citation6–16], dopamine receptor agonist [Citation17], cardiotonic [Citation18], antihypertensive [Citation19], and antiulcer [Citation20]. In our laboratory, we have designed and synthesized some 2-benzoxazolinone derivatives in the search for new non-steroidal anti-inflammatory agents [Citation6–9, Citation12, Citation14]. A considerable number of the prepared compounds have been found to have analgesic-anti-inflammatory activity comparable to or higher than that of indomethacine. In this paper, we report the synthesis and pharmacological screening of a new series of 2(3H)-benzoxazolones in order to go deep into structure-activity relationships for further studies. On this basis we have synthesized some 5-methyl-3-benzoylmethyl-2(3H)-benzoxazolones, reduced derivatives of them 5-methyl-3-(2-hydroxyl-2-phenylethyl)-2(3H)-benzoxazolones and 6-(difluorobenzoyl)-3-benzoylmethyl-2(3H)-benzoxazolones. The synthesized compounds were tested for their analgesic and antinflammatory activities.
Experimental
Chemistry
Melting points were determined through a Thomas Hoover capillary melting point apparatus and are uncorrected. Infrared (IR) spectra were obtained with a Bruker Vector 22 IR (Opus Spectroscopic Software Version 2.0) spectrometer by using potassium bromide plates and expressed in wave number (cm− 1). Nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were scanned on a Bruker 80 MHz spectrometer using chloroform as solvent. Chemical shifts are expressed in δ (parts per million) relative to tetramethylsilane. The mass spectra were obtained with electron impact technique by a Direct Insertion Probe and Agilent 5973-Network Mass Selective Dedector at 70 eV. Elemental analyses (C, H, N) were performed on Leco CHNS 932 analyzer.
Synthesis of 5-methyl-2(3H)-benzoxazolone
5-Methyl-2(3H)-benzoxazolone was synthesized as a result of the reaction of 5-methyl-2-hydroxyaniline with urea in oil bath according to the method reported earlier [Citation21].
5-methyl-3-benzoylmethyl-2(3H)-benzoxazolone (Compound 3a–3d)
To the solution of sodium 5-methyl-2(3H)-benzoxazolones in alcohol, equimolar α-bromo-4-substituted acetophenone was added. The solution was refluxed on an oil bath for 4 h and then cooled. The crude product was filtered, washed with water, dried and crystallized from a suitable solvent.
6-Difluorobenzoyl-2(3H)-benzoxazolone
6-Difluorobenzoyl-2(3H)-benzoxazolone was synthesized by treating 2(3H)-benzoxazolone and difluorobenzoic acid in polyphosphoric acid by the method reported earlier [Citation22].
5-methyl-3-(2-hydroxyl-2-phenylethyl)-2(3H)-benzoxazolone (Compound 3e–3h, 4a–4d)
0.01 mol 5-methyl-3-benzoylmethyl-2(3H)-benzoxazolone was dissolved in methanol. To this mixture 0.012 ml of NaBH4 was added, and stirring was continued for 4 h at reduced pressure. The residue was recrystallized from different solvents.
5-methyl-3-benzoylmethyl-2(3H)-benzoxazolone (3a)
Yield: 32%, mp 125°C. IR (KBr): ν 3061, 2967, 2927, 1774, 1689. 1H-NMR (80 MHz, CDCl3): δ 2.2 (3H, s, CH3), 5.1 (2H, s, N–CH2–), 6.5–8.0 (8H, m, Arom.H). EI (70 eV): m/z: 267 (M+), 162, 105, 77. Analysis calculated for C16H13NO3 (267.28) Anal. found: C, 71.7; H, 4.98; N, 5.51; Anal. calcd: C, 71.9; H, 4.90; N, 5.24%.
5-methyl-3-(4-methylbenzoylmethyl)-2(3H)-benzoxazolone (3b)
Yield: 40%, mp 159°C. IR (KBr): ν 3054, 2969, 2921, 1763,. 1H-NMR (80 MHz, CDCl3): δ 2.2 (3H, s, CH3-phenyl), 2.3 (3H, s, CH3); 5.1 (2H, s, N–CH2–); 6.5–7.9 (7H, m, Arom.H). EI (70 eV): m/z: 281 (M+), 119, 91. Analysis calculated for C17H15NO3 (281.31) Anal. found: C, 72.69; H, 4.85; N, 4.94; Anal. calcd: C, 72.58; H, 5.37; N, 4.98%.
5-methyl-3-(4-bromobenzoylmethyl)-2(3H)-benzoxazolone (3c)
Yield: 40%, mp 171°C. IR (KBr): ν 2963, 2931, 1759, 1696. 1H-NMR (80 MHz, CDCl3): δ 2.3 (3H, s, CH3), 5.1 (2H, s, N–CH2–), 6.6–8.0 (7H, m, Arom.H). EI (70 eV): m/z: 345 (M+), 347 (M + 2), 183, 185 (base peak), 162,91. Analysis calculated for C16H12BrNO3 (346.18) Anal. found: C, 63.76; H, 3.46; N, 4.63; Anal. calcd: C, 63.69; H, 4.01; N, 4.64%.
5-methyl-3-(4-chlorobenzoylmethyl)-2(3H)-benzoxazolone (3d)
Yield: 56%, mp 164°C. IR (KBr): ν 3040, 2964, 2928, 1758, 1694. 1H-NMR (80 MHz, CDCl3): δ2.3 (3H, s, CH3), 5.1 (2H, s, N–CH2–), 6.6–8.05 (7H, m, Arom.H). EI (70 eV): m/z: 301 (M+), 303 (M + 2), 162, 139, 141, 111, 113, 91. Analysis calculated for C16H12ClNO3 (301.73) Anal. found: C, 63.76; H, 3.46; N, 4.63; Anal. calcd: C, 63.69; H, 4.01; N, 4.64%.
6-(2,5-Difluorobenzoyl)-3-(4-bromobenzoylmethyl)-2(3H)-benzoxazolone (3e)
Yield: 85%, mp 217°C, Ref [Citation22].
6-(2,5-Difluorobenzoyl)-3-(4-chlorobenzoylmethyl)-2(3H)-benzoxazolone (3f)
Yield: 74%, mp 227°C, Ref [Citation22].
6-(2,6-Difluorobenzoyl)-3-(4-bromobenzoylmethyl)-2(3H)-benzoxazolone (3g)
Yield: 67%, mp 240°C, Ref [Citation22].
6-(2,6-Difluorobenzoyl)-3-(4-chlorobenzoylmethyl)-2(3H)-benzoxazolone (3e)
Yield: 77%, mp 217°C, Ref [Citation22].
5-methyl-3-(2-hydroxyl-2-phenylethyl)-2(3H)-benzoxazolone (4a)
Yield: 99%, mp 103–4°C. IR (KBr): ν 3467, 1750. 1H-NMR (80 MHz, CDCl3): δ 2.3 (3H, s, CH3); 3.95 (2H, d, N–CH2–); 5.05 (1H, t, CH2–CH), 6.8–7.6 (8H, m, Arom.H). EI (70 eV): m/z: 163, 134, 121, 91, 77. Analysis calculated for C16H15NO3 (269.30) Anal. found: C, 71.48; H, 5.96; N, 4.92; Anal. calcd: C, 71.36; H, 5.61; N, 5.20%.
5-methyl-3-[2-hydroxyl-2-(4-methylphenyl)ethyl]-2(3H)-benzoxazolone (4b)
Yield: 92%, mp 105–6°C. IR (KBr): ν 3450, 1750. 1H-NMR (80 MHz, CDCl3): δ2.3 (6H, s, CH3, CH3-fenil); 4.0 (2H, d, N–CH2–); 5.05 (1H, t, CH2–CH), 6.7–7.5 (7H, m, Arom.H). EI (70 eV): m/z: 163, 134, 121, 91, 77. Analysis calculated for C17H17NO3 (283.33) Anal. found: C, 72.03; H, 5.46; N, 4.93; Anal. calcd: C, 72.07; H, 6.05; N, 4.94%.
5-methyl-3-[2-hydroxyl-2-(4-bromophenyl)ethyl]-2(3H)-benzoxazolone (4c)
Yield: 99%, mp 148–9°C. IR (KBr): ν 3430, 1745. 1H-NMR (80 MHz, CDCl3): δ 2.3 (3H, s CH3); 4.0 (2H, d, N–CH2–); 5.1 (1H, t, CH2–CH), 6.7–7.6 (7H, m, Arom.H). EI (70 eV): m/z: 347 (M+), 348 (M + 2), 185, 187, 163, 134, 91, 77. Analysis calculated for C16H14BrNO3 (348.20). Anal. found: C, 55.46; H, 4.26; N, 4.32; Anal. calcd: C, 55.19; H, 4.05; N, 4.02%.
5-methyl-3-[2-hydroxyl-2-(4-chlorophenyl)ethyl]-2(3H)-benzoxazolone (4d)
Yield: 97%, mp 139–140°C. IR (KBr): ν 3463, 1742. 1H-NMR (80 MHz, CDCl3): δ 2.3 (3H, s, CH3); 4.0 (2H, d, N–CH2–); 5.1 (1H, t, CH2–CH), 6.7–7.5 (7H, m, Arom.H). EI (70 eV): m/z: 303 (M+), 305 (M + 2), 163, 134, 91, 77. Analysis calculated for C16H14ClNO3 (303.75).Anal. found: C, 63.58; H, 4.99; N, 4.63; Anal. calcd: C, 63.27; H, 4.65; N, 4.61%.
Pharmacology
Animals
Male Swiss albino mice (20–25 g) were purchased from the animal breeding laboratories of Fırat University (Elazıg, Turkey). Before treatments, the animals were acclimatized to animal laboratory conditions for two days and were fed on standard pellet diet and water ad libitum. On the day before the treatments, the food was withdrawn, but the animals were allowed free access of water. A minimum of five animals was used in each group, otherwise it is described in the procedure. Mice used in the present study were cared for in accordance with the directory of Fırat University Animal Care Unit, which applies the guidelines of National Institutes of Health on laboratory animal welfare.
Preparation of test samples for bioassay
Test samples were given orally to test animals after suspending in 0.5% sodium carboxymethyl cellulose (CMC) and distilled water. The control group animals received the same experimental handling as those of the test groups except that the drug treatment was replaced with appropriate volumes of the dosing vehicle. Either indomethacin (10 mg/kg), or acetylsalicylic acid (ASA) (200 mg/kg) in 0.5% CMC was used as reference drug.
Anti-inflammatory activity
Carrageenan induced oedema [Citation23]
For the determination of the effects on carrageenan-induced paw oedema the modified method of Kasahara et al. was employed [Citation23]. One hour after the oral administration of either test sample or dosing vehicle, each mice was injected with freshly prepared (0.5 mg/25 μL) suspension of carrageenan (Sigma, St. Louis, Missouri, U.S.A.) in physiological saline (154 mM NaCl) into subplantar tissue of the right hind paw. As the control, 25 μL saline solution was injected into that of the left hind paw. Paw oedema was measured in every 90 min during 6 h after induction of inflammation. The difference in footpad thickness between the right and left foot was measured with a pair of dial thickness gauge callipers (Ozaki Co., Tokyo, Japan). Mean values of treated groups were compared with mean values of a control group and analyzed using statistical methods. Indomethacin was used as a reference compound and administered at 10 mg/kg.
Analgesic activity
Koster test [Citation24]
One hour after the oral administration of test sample, each mouse was injected with 3% (w/v) acetic acid solution (0.1 mL/10 g body weight) intraperitoneally. Starting 5 min after the acetic acid injection, the number of muscular contractions on mice were counted for a period of 10 min. A significant reduction in the number of writhings by any treatment as compared to control animals was considered as a positive analgesic response. The antinociceptive activity was expressed as percentage change from writhing controls. Percent inhibitory effects were estimated according to the following equation, where n was the average difference in thickness between the left and right hind paw of control group and n′ was that of test group of animals. Aspirin (ASA) was used as a reference compound and administered at 200 mg/kg.
Constant temperature hot-plate test [Citation25]
An HTC Inc. Mod. 35-D analgesiameter was set to give a plate temperature of 54 ± 0.5°C. One hour after oral administration of the compounds (100 mg/kg), the animals were placed on the hot-plate and confined by a lidded perspex box in a compartment measuring 13.8 × 13.8 cm, and the latency to the first hind-paw lick was recorded. If no hind-paw lick occurred, the test was terminated after 30 s. Morphine was used as a reference analgesic and administered i.p. at 10 mg/kg.
Statistical analysis of data
Data obtained from animal experiments were expressed as means ± standard error (SEM). Differences between the control and treated groups were tested for significance using a one-way analysis of variance (ANOVA), followed by Dunnett's t-test.
Result and discussion
Chemistry
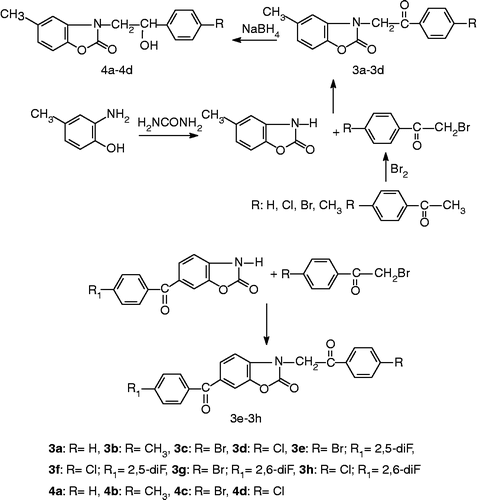
5-Methyl-3-(4-substituted benzoylmethyl)-2(3H)-benzoxazolonones (3a–3d) were prepared by reacting 5-methyl-2(3H)-benzoxazolones, α-bromo-4-substituted acetophenones and sodium hydroxide in ethanol. Compounds 4a–4d were obtained by the reduction of ketones (3a–3d) with NaBH4. Compounds 4a–4d have one asymmetric carbon atom. However the enantiomers were not separated. Studies directed toward separation of enantiomers will be done in the near future. 6-Acyl-2(3H)-benzoxazolones were prepared by reacting 2(3H)-benzoxazolone with difluorobenzoic acids in the presence of polyphosphoric acid at 140–160°C. The other compounds 3e–3h were prepared from 6-acyl-2(3H)-benzoxazolone and α-bromo-4-substituted acetophenone [Citation22]. The synthetic pathway for preparation of the targeted componds is shown in .
The structure of the compounds was confirmed by IR, 1H-NMR, Mass and elemental analysis. In IR spectra of the compounds, the bands seen at 1742–1774 cm− 1 (lactam C = O) and 1682–1696 cm− 1 (CH2–C = O) were in accordance with the assumed structures. Furthermore, N–H stretching bands belonging to 2(3H)-benzoxazolone ring disappeared with the reaction of benzoylmethyl bromides. The formation of compounds 4a–4d were confirmed by the presence of OH signals at 3030–3070 cm− 1 in the IR spectra.
In the 1H-NMR spectra of the compounds, 5-methyl protons bound to 2(3H)-benzoxazolone appeared at approximately 2.2–2.3 ppm. The methylene protons seen as singlet at 5.1 ppm proved the presence of benzoylmethyl moiety. The same methylene protons were observed in 3.95–4.0 ppm in reduced derivatives 4a–4d. The triplets of (–CH) methyne protons of the compounds were found to be 5.05–5.1 ppm.
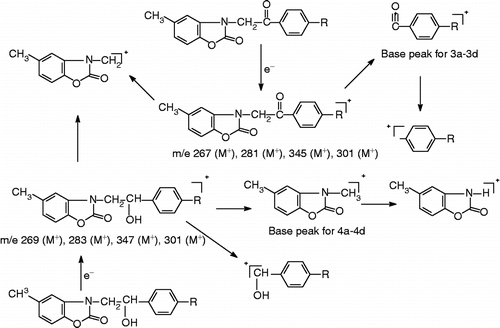
The mass spectra of the compounds were studied under electron ionization. Molecular ion M+ peaks which appeared at different intensities confirmed the molecular weights of the examined compounds except compound 4a. Characteristic M + 2 isotop peaks are observed in the mass spectra of the compounds having a halogen. In the spectra of compounds 3a–3d, the base peak resulted from loss of N,5-dimethyl-2(3H)-benzoxazolone while in the spectra of compound 4a–4d the lost group was the base peak (). In elemental analysis the results support the structures with ± 0.4% of the theoretical values.
Pharmacology
Antiinflammatory activity
In order to screen the anti-inflammatory profile of the synthesized compounds, carrageenan-induced hind paw oedema model in mice was used [Citation23]. Since the carrageenan oedema has been used in the development of indomethacine, many researchers have adapted this procedure for screening potential anti-inflammatory compounds. Carrageenan-induced oedema is a nonspecific inflammation maintained by the release of histamine, 5-hydroxytryptamine, kinins and later by prostaglandins [Citation26]. The inhibitory effect of NSAIDs, such as indomethacin, is usually weak in the first phase (1–2 h), in contrast with their strong inhibition in the second phase (3–4 h) [Citation27].
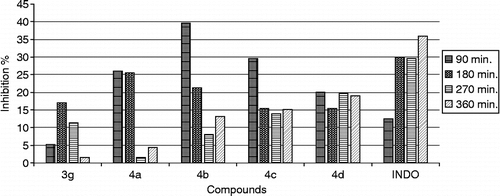
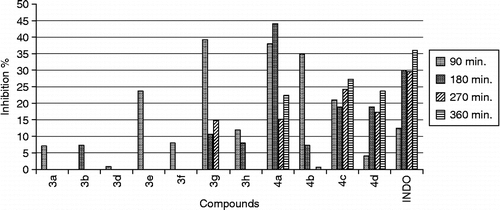
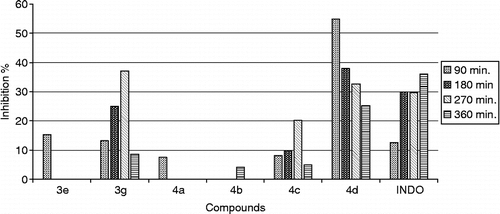
The anti-inflammatory activity of the synthesized compounds was studied at 100 mg/kg dose. Test compounds which possessed more than 20% effect, even some of them are not be significant statistically, were considered for further evaluation and the experiments were repeated for the compounds in two different dose levels (50 and 200 mg/kg). A notable decrease in activity was seen compounds 3a–3d bearing 4-substituted benzoylmethyl in the third position of 2(3H)-benzoxazolone. When compared the ketone and alcohol groups, It was seen that reduced derivatives (4a–4d) were more effective than those of ketone (3a–3d) (, ).
Table I. Analgesic/anti-inflammatory activities for compounds 3a–3h, 4a–4d.
Good inhibition of the second phase of carrageenan-induced oedema was observed for the compounds 4a, 4c, 4d tested, suggesting that they interfere with prostaglandin synthesis. 90 and 180 minutes after the administration of the drug, compound 4a bearing no substituent at the phenyl ring exhibited much significant activity at 50 and 100 mg/kg dose when compared to indomethacin. However, 360 minutes after the administration of the drug, the same compound 4a showed half activity of that of indomethacin at 100 mg/dose. It was shown that compound 4c carrying brom substituent at 100 mg/kg dose and compound 4d bearing chloro substituent at 200 mg/kg dose have more significant activity among the reduced derivatives.
Analysis of the activities of the compounds 3e–3h bearing substituted benzoyl methyl in 3 position and difluorobenzoyl in 6 position of the 2(3H)-benzoxazolone moiety showed the difference in the antiinflammatory action due to the substituents in the benzoylmethyl group and position of fluors in the benzoyl group.. As a result of this, the compounds possessing 4-bromobenzoylmethyl and 2,6-difluorobenzoyl moieties were more active than the others including 2,5-difluorobenzoyl and 4-chlorobenzoylmethyl ().
Analgesic activity
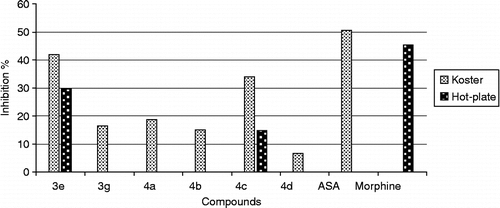
The analgesic activity of the compounds were studied by using both the acetic acid-induced writhing test [Citation24] and hot plate test [Citation25] in mice. The animals were first administered in 100 mg/kg (body weight) dose of the test drugs in two of the screening tests. Test compounds which possessed more than 20% effect at 100 mg/kg dose were evaluated for analgesic activity (, ). Among the six derivatives investigated, two compounds (3e, 4c) showed high activity. According to this, the compound 3e possessing 2,5-difluorobenzoyl in 6 position and 4-bromobenzoyl methyl in the third position of 2(3H)-benzoxazolone ring is the most active derivative (41.7%). Most of the structure-activity relationships seen for the anti-inflammatory activity were not confirmed for the analgesic activity. The analgesic activity of reduced derivatives 4a, 4b, 4d were not significant when compared to the activity obtained by Aspirin. The mouse abdominal constriction test can be used to detect both peripherally and centrally acting analgesics, whereas the hot-plate test has been demonstrated to be predictive of centrally acting analgesics. Therefore the analgesic activity of the two compounds was also determined by hot-plate test. Constant temperature hot-plate test result () was in good accordance with the Koster test for compound 3e. It showed moderately significant activity.
Acknowledgements
This study was supported by the Hacettepe University Research Center Fund (Project number: 05 D 301002).
References
- G Dannhardt, and W Kiefer. (2001). Cyclooxygenase inhibitors-current status and future prospects. Eur J Med Chem 36:109–126.
- TD Penning, JJ Talley, SR Bertenshaw, JS Carter, PW Collins, S Docter, MJ Graneto, LF Lee, JW Malecha, JM Miyashiro, RS Rogers, DJ Rogier, SS Yu, GD Anderson, EG Burton, JN Cogburn, SA Gregory, CM Koboldt, WE Perkins, K Seibert, AW Veenhuizen, YY Zhang, and PC Isakson. (1997). Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib). J Med Chem 40:1347–1365.
- P Prasit, Z Wang, C Brideau, CC Chan, S Charleson, W Cromlish, D Ethier, JF Evans, AW Ford-Hutchinson, JY Gauthier, R Gorgon, J Guay, M Gresser, S Kargman, B Kennedy, Y Leblanc, S Léger, J Mancini, GP O'Neill, M Ouellet, MD Percival, H Perrier, D Riendeau, I Rodger, P Tagari, M Thérien, P Vickers, E Wong, LJ Xu, RN Young, and R Zamboni. (1999). The discovery of rofecoxib [MK 966, VIOXX®, 4-(4′-methylsulfonylphenyl)-3-phenyl-2(5H)-furanone], an orally active cyclooxygenase-2 inhibitor. Bioorg Med Chem Lett 9:1773–1778.
- P Jüni, L Nartey, S Reichenbach, R Sterchi, PA Dieppe, and M Egger. (2004). Risk of cardiovascular events and rofecoxib: Cumulative meta-analysis. Lancet 364:2021–2029.
- Alert for Healthcare Professionals Valdecoxib (marketed as Bextra). Available at: http://www.fda.gov/cder/drug/InfoSheets/HCP/valdecoxibHCP.htm. Accessed June 24, 2005.
- H Erdoğan, M Debaert, and J Cazin. (1991). Synthesis of some 2-benzoxazolinone derivatives and their analgesic properties. Arzneim-Forsch/Drug Res 41:73–76.
- E Palaska, S Ünlü, F Özkanlı, G Pilli, H Erdoğan, C Şafak, R Demirdamar, and B Gümüşel. (1995). 3-substituted piperazinomethyl benzoxazolinones: Analgesic and antiinflammatory compounds inhibiting prostaglandin E2. Arzneim-Forsch/Drug Res 45:693–697.
- N Gökhan, H Erdoğan, BC Tel, and R Demirdamar. (1996). Analgesic and antiinflammatory activity screening of 6-acyl-3-piperazinomethyl-2-benzoxazolinone derivatives. Eur J Med Chem 31:625–628.
- N Gökhan, H Erdoğan, NT Durlu, and R Demirdamar. (1999). Novel antiinflammatory analgesics: 3-(2-/4-Pyridylethyl)-benzoxazolinone and oxazolo[4,5-b]pyridin-2-one derivatives. Arch Pharm 332:43–49.
- S Unlu, T Onkol, Y Dundar, B Okcelik, E Kupeli, E Yesilada, N Noyanalpan, and MF Sahin. (2003). Synthesis and analgesic and anti-inflammatory activity of some new (6-acyl-2-benzoxazolinone and 6-acyl-2-benzothiazolinone derivatives with acetic acid and propanoic acid residues. Arch Pharm 336:353–360.
- E Banoglu, B Okcelik, E Kupeli, S Unlu, E Yesilada, M Amat, JF Caturla, and MF Sahin. (2003). Amide derivatives of [5-chloro-6-(2-chloro/fluorobenzoyl)-2-benzoxazolinone-3-yl]acetic acids as potential analgesic and anti-inflammatory compounds. Arch Pharm 336:251–257.
- N Gökhan, H Erdoğan, NT Durlu, R Demirdamar, and M Özalp. (2003). Synthesis and evaluation of analgesic, antiinflammatory and antimicrobial activities of 6-acyl-3-piperazinomethyl-2-benzoxazolinones. Arzneim-Forsch/Drug Res 53:114–120.
- Z Soyer, M Bas, A Pabuççuoğlu, and V Pabuççuoğlu. (2005). Synthesis of some 2(3H)-benzoxazolone derivatives and their in vitro effects on human leukocyte myeloperoxidase activity. Arch Pharm 338:405–410.
- M Köksal, N Gökhan, E Küpeli, E Yeşilada, and H Erdoğan. (2005). Synthesis, analgesic and antiinflammatory of certain 5-/6-acyl-3-(4-substituted-1-piperazinylmethyl)-2-benzoxazolinones derivatives. Arch Pharm 338:117–125.
- B Ökçelik, S Ünlü, E Banoğlu, E Küpeli, E Yeşilada, and MF Şahin. (2003). Investigations of new pyridazinone derivatives for the synthesis of potent analgesic and anti-inflammatory compounds with cyclooxygenase inhibitory activity. Arch Pharm 336:406–412.
- S Ünlü, S Nacak Baytaş, E Küpeli, and E Yeşilada. (2003). Studies on novel 7-acyl-5-chloro-2-oxo-3H-benzoxazole derivatives as potential analgesic and anti-inflammatory agents. Arch Pharm 336:310–321.
- J Weinstock, DE Gaitanopoulos, OD Stringer, RG Franz, JP Hieble, LB Kinter, WA Mann, KE Flaim, and G Gessner. (1987). Synthesis and evaluation of non-catechol D-1 and D-2 dopamine receptor agonist: Benzimidazol-2-one, benzoxazol-2-one and the highly potent benzothiazol-2-one 7-ethylamines. J Med Chem 30:1166–1176.
- W Von der Saal, JP Hölck, W Kampe, A Metens, and B Müller-Beckmann. (1989). Nonsteroidal cardiotonics 2. The initropic activity of linear tricycic 5,6,5 fused heterocycles. J Med Chem 32:1481–1491.
- JP Bonte, JP Piancastelli, MCI Lesieur, JC Lamar, M Beaughard, and G Dureng. (1990). Amino ketone and amino alcohol derivatives of benzoxazolinone: Synthesis, adrenergic and antihypertensive properties. Eur J Med Chem 25:361–368.
- Y Katsura, S Nishino, and H Takasugi. (1991). Studies on antiulcer drugs. I. Synthesis and antiulser activities of imidazo[1,2-a]pyridinyl-2-oxobenzoxazolidines-3-oxo-1,4-benzoxazines and related compounds. Chem Pharm Bull 39:2937–2943.
- WJ Close, BD Tiffany, and MA Spielman. (1949). The analgesic activity of some benzoxazolone derivatives. J Am Chem Soc 71:1265–1268.
- M Köksal, N Gökhan, H Erdoğan, M Özalp, and M Ekizoğlu. (2002). Synthesis of 3-(4-substituted benzoylmethyl)-2-benzoxazolinones and screening antimicrobial activities. II Farmaco 57:535–538.
- Y Kasahara, H Hikino, S Tsurufuji, M Watanabe, and K Ohuchi. (1985). Antiinflammation actions of ephedrines in acute inflammations. Planta Med 51:325–331.
- R Koster, M Anderson, and EJ de Beer. (1959). Acetic acid for analgesic screening. Fed Proc 18:412.
- JH Rosland, SO Hunskaar, OJ Broch, and K Hole. (1992). Acute and long term effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in tests of nociception in mice. Pharmacol Toxicol 70:31–37.
- K Tsurumi, K Kyuki, M Niwa, S Kokuba, and H Fujimura. (1986). Pharmacological investigations of the new antiinflammatory agent 2-(10,11-dihydro-10-oxodibenzo[b,f]thiepin-2-yl)propionic acid. Arzneim Forsch/Drug Res 36:1796–1803.
- A Gavalas, L Kourounakis, D Litina, and P Kourounakis. (1991). Anti-inflammatory and immuno-modulating effects of the novel agent γ-(2-aminoethylamino)-2-butyrothienone. Inhibitory effects on mouse paw oedema. Arzneim Forsch/Drug Res 41:423–426.