Abstract
New 3-chloro-1-hydroxy-2,6-diarylpiperidin-4-ones 18–22 were synthesized, characterized by melting point, elemental analysis, MS, FT-IR, one-dimensional NMR (1H & 13C) spectroscopic data and evaluated for their in vitro antibacterial and antifungal activities. All the newly synthesized compounds exerted a wide range of antibacterial activities against the entire tested gram-positive and gram-negative bacterial strains except Escherichia coli. Compounds 21 and 22 exerted strong antifungal activities against Aspergillus flavus, mucor and Microsporum gypsuem. In addition, compound 20 was more potent against Rhizopus.
Introduction
Now-a-days, bioactive heterocyclic ring systems having 2,6-diaryl-piperidine-4-one nucleus with different substituents at 3- and 5-positions of the ring have aroused great interest due to their wide variety of biological properties such as antiviral, antitumour [Citation1,Citation2], central nervous system [Citation3], local anesthetic [Citation4], anticancer [Citation5], and antimicrobial activity [Citation6] and their derivative piperidine are also biologically important and act as neurokinin receptor antagonists [Citation7], analgesic and anti-hypertensive agents [Citation8]. But, only very few reports [Citation9,Citation10] are available with chloro substitution at position 3.
In addition, hydroxylamines have been reported as anti-bacterial, antifungal and antileukemic agents. N-Hydroxy urea was one of the effective antineouplasmic agents [Citation11] and ciclopirox has broad-spectrum antifungal activity [Citation12]. N-hydroxy pyrrolizidine alkaloid intermediate is used for the synthesis of dl-retronecine [Citation13], the most widely occurring of the necine bases exhibits marked hepatoxic and antitumour properties.
Due to an increase in the number of immuno-compromised hosts, [Citation14], over the past decades, the incidence of systemic microbial infections has been increasing dramatically. The increasing incidence of bacterial resistance to a large number of antibacterial agents such as glycopeptides (vancomycin, inhibition cell walls synthesis), sulfonamide drugs (inhibitors of tetrahydrofolate synthesis), β-lactam antibiotics (penicillins and cephalosporins), nitroimidazoles and quinolones (DNA inhibitors), tetracyclins, chloramphenicoland macrolides (erythromycin, inhibiting protein synthesis) is becoming a major concern [Citation15]. For the past several years, vancomycin has been considered the last line of defense agent against Gram-positive infections and no alternative drugs for treating diseases that have become resistant to vancomycin [Citation16]. Patients undergoing organ transplants, anticancer chemotherapy or long treatment with antimicrobial agents and patients with AIDS are immuno suppressed and very susceptible to life threatening systemic fungal infections like Candidiasis, Cryptococcosis and Aspergillosis. Antifungal azoles, fluconazole and itraconazole which are strong inhibitors of lanosterol 14α-demethylase (cytochrome P45014DM) and orally active have been widely used in antifungal chemotherapy. Reports are available on the developments of resistance to currently available antifungal azoles in Candida spp., as well as clinical failures in the treatment of fungal infections Citation17–20. Furthermore, most of the present antifungal drugs are not effective against invasive Aspergillosis and the only drug of choice in such patients is the injectable amphotericin B. These observations places new emphasis on the need of as well as search for alternative new and more effective antimicrobial agents with a broad spectrum.
In the course of broad programme in developing biologically active molecules, we have recently reported the synthesis of 2,6-diarylpiperidin-4-one derivatives and evaluated their biological importance Citation21–23. Therefore it was planned to synthesize a system which combines these two biologically active components (3-chloro-piperidin-4-one and hydroxyl amine) together to give a combined structure like the title compound. Inorder to extend our knowledge in structure-activity relationship, all the newly synthesized compounds are tested for their in vitro antibacterial and antifungal activities and the influence of some structural variations by varying the substituents at the phenyl ring in the synthesized compounds towards their biological activities is evaluated.
Experimental
Chemistry
TLC was performed to assess the reactions and the purity of the products. All the reported melting points were taken in open capillaries and were uncorrected. IR spectra were recorded in KBr (pellet forms) on a Nicolet-Avatar–330 FT-IR spectrophotometer and note worthy absorption values (cm− 1) alone are listed. 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz respectively on Bruker AMX 400 NMR spectrometer using CDCl3 as solvent. The ESI + ve MS spectra were recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis was obtained on Carlo Erba 1106 CHN analyzer.
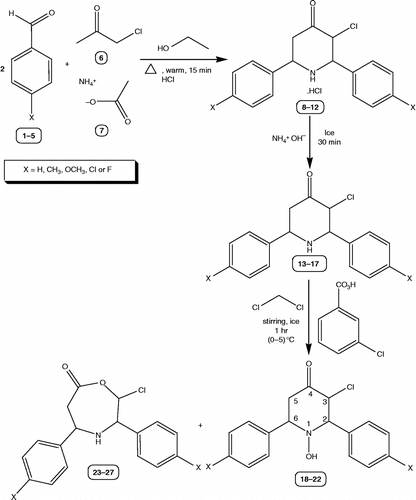
By adopting the literature precedent [Citation9], 3-chloro-2,6-diarylpiperidin-4-ones were prepared 13–17.
Synthesis of 3-chloro-1-hydroxy-2,6-diphenylpiperidin-4-one 18:
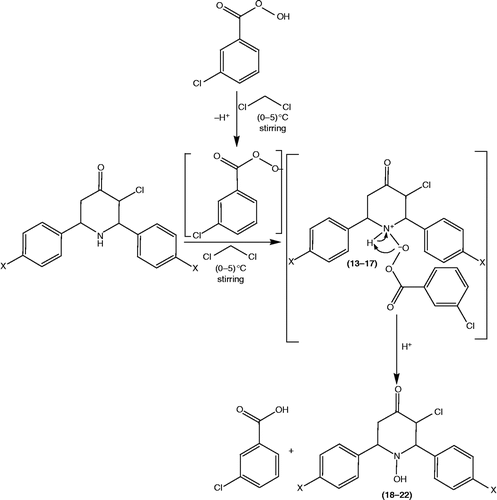
A solution of 3-chloro-2,6-diphenylpiperidin-4-one 13 (0.001 mol) and m-chloroperbenzoic acid (0.001 mol) in 50 mL of dichloromethane was stirred for 1 h at (0–5)oC and kept aside for overnight at 20oC. Then the mixture was extracted with dichloromethane and washed with 10% sodium bicarbonate solution. The dichloromethane layer was dried over anhydrous sodium sulphate and distilled off under reduced pressure. Purifications by silica gel column chromatography with ethyl acetate: petroleum ether (bp60–80) 2:8 mixture yielded the product 3-chloro-1-hydroxy-2,6-diarylpiperidin-4-ones 18. IR (KBr) (cm− 1): 3492, 3033, 2922, 2852, 1729, 753, 698; 1H NMR (δ ppm): 2.80–2.88 (m, 1H, H5a); 2.95–3.01 (m, 1H, H5e), 3.99 (d, 1H, H2a, J = 11.3), 4.07–4.36 (m, 1H, H6a), 4.55 (s, 1H, H1), 4.72 (d, 1H, H3a, J = 11.0), 7.26–7.51 (m, 10H, Harom); 13C NMR (δ ppm): 46.7 C-5, 65.7 C-3, 67.6 C-6, 70.1 C-2, 128.1–128.9 –Carom, 139.0, 140.63 ipso-C, 196.7 C-4.
The compounds 19–22 were synthesized similarly.
3-Chloro-1-hydroxy-2,6-bis(p-methylphenyl)piperidin-4-one 19:
IR (KBr) (cm− 1): 3485, 3026, 2921, 2859, 1728, 787, 677; 1H NMR (δ ppm): 2.35 (s, 6H, CH3 at phenyl rings), 2.80–2.91 (m, 1H, H5a); 2.95–2.98 (m, 1H, H5e), 3.93 (d, 1H, H2a, J = 11.1), 3.98–4.29 (m, 1H, H6a), 4.50 (s, 1H, H1), 4.69 (d, 1H, H3a, J = 11.1), 7.18–7.40 (m, 8H, Harom); 13C NMR (δ ppm): 21.0 CH3 at phenyl rings, 46.7 C-5, 67.2 C-3, 69.8 C-6, 70.5 C-2, 126.7–129.3 –Carom, 129.5, 136.01, 137.89, 138.2 ipso-C, 196.8 C-4.
3-Chloro-1-hydroxy-2,6-bis(p-methoxyphenyl)piperidin-4-one 20:
IR (KBr) (cm− 1): 3495, 3006, 2964, 2934, 2840, 1727, 745, 671; 1H NMR (δ ppm): 2.80–2.91 (m, 1H, H5a); 2.95–2.98 (m, 1H, H5e), 3.80 (s, 6H, OCH3 at phenyl rings), 3.90 (d, 1H, H2a, J = 11.1), 3.95–4.14 (m, 1H, H6a), 4.47 (s, 1H, H1), 4.67 (d, 1H, H3a, J = 11.1), 7.26–7.53 (m, 8H, Harom); 13C NMR (δ ppm): 46.7 C-5, 53.40, 55.27 OCH3 at phenyl rings, 66.0 C-3, 67.0 C-6, 70.2 C-2, 128.03–130.4 –Carom, 131.0, 132.6, 159.3, 159.5 ipso-C, 196.8 C-4.
3-Chloro-1-hydroxy-2,6-bis(p-chlorophenyl)piperidin-4-one 21:
IR (KBr) (cm− 1): 3483, 2983, 2923, 2874, 1736, 826, 803, 666; 1H NMR (δ ppm): 2.82–2.85 (m, 1H, H5a); 2.88–2.95 (m, 1H, H5e), 3.96 (d, 1H, H2a, J = 11.0), 4.01–4.07 (m, 1H, H6a), 4.53 (s, 1H, H1), 4.64 (d, 1H, H3a, J = 11.3), 7.26–7.48 (m, 8H, Harom); 13C NMR (δ ppm): 46.7 C-5, 65.7 C-3, 67.6 C-6, 70.1 C-2, 126.9–128.5 –Carom, 128.6, 128.9, 139.0, 140.6 ipso-C, 196.7 C-4.
3-Chloro-1-hydroxy-2,6-bis(p-fluorophenyl)piperidin-4-one 22:
IR (KBr) (cm− 1): 3482, 3044, 2923, 2874, 1735, 755, 676; 1H NMR (δ ppm): 2.82–2.85 (m, 1H, H5a); 2.90–2.97 (m, 1H, H5e), 3.96 (d, 1H, H2a, J = 11.6), 4.00–4.14 (m, 1H, H6a), 4.50 (s, 1H, H1), 4.65 (d, 1H, H3a, J = 11.1), 7.06–7.48 (m, 8H, Harom);13C NMR (δ ppm): 46.6 C-5, 66.8 C-3, 68.0 C-6, 68.3 C-2, 128.2-129.7 –Carom, 131.0, 134.6, 163.7, 163.8 ipso-C, 196.0 C-4.
Microbiology
Materials
All the bacterial strains namely Staphylococcus aureus, β-Haemolytic streptococcus, Vibreo cholerae, Salmonella typhii, Shigella felxneri, Escherichia coli, Klebsiella pneumonia, Pseudomonas and fungal strains namely Aspergillus flavus, Mucor, Rhizopus and Microsporum gypsuem were obtained from Faculty of Medicine, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India.
In vitro antibacterial and antifungal activity
The in vitro activities of the compounds were tested in Sabourauds dextrose broth (SDB) (Hi-media, Mumbai) for fungi and nutrient broth (NB) (Hi-media, Mumbai) for bacteria by two-fold serial dilution method [Citation26]. The respective test compounds 18–22 were dissolved in dimethylsulfoxide to obtain 1 mg ml− 1 stock solution. Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1 to 7 days old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in SDB. The colony forming units (cfu) of the seeded broth were determined by plating technique and adjusted in the range of 104–105 cfu/mL. The final inoculums size was 105cfu/mL for antibacterial assay and 1.1–1.5 × 102 cfu/mL for antifungal assay. Testing was performed at pH 7.4 ± 0.2 for bacteria (NB) and at a pH 5.6 for fungi (SDB). Exactly 0.4 mL of the solution of test compound was added to 1.6 mL of seeded broth to form the first dilution. One milliliter of this was diluted with a further 1 mL of seeded broth to give the second dilution and so on till six such dilutions were obtained. A set of assay tubes containing only seeded broth was kept as control. The tubes were incubated in BOD incubators at 37 ± 1°C for bacteria and 72–96 h for fungi. The minimum inhibitory concentrations (MICs) were recorded by visual observations after 24 h (for bacteria) and 72–96 h (for fungi) of incubation. Ciprofloxacin was used as standard for bacteria studies and Fluconazole was used as standards for fungal studies.
Results and discussion
Chemistry
Target molecules, 3-Chloro-1-hydroxy-2,6-diarylpiperidin-4-ones 18–22 were synthesized as a result of a three-step synthetic strategy. One of the direct synthetic route for the formation of 3-chloro-2,6-diarylpiperidin-4-ones 13–17 is as follows: A mixture of chloroacetone 6, appropriate benzaldehyde 1–5 and ammonium acetate 7 in the ratio of 1:2:1, was warmed for 15 min. and hydrochloric acid was added to afford 3-chloro-2,6-diaryl-piperidin-4-ones hydrochloride 8–12, which upon neutralization with aqueous ammonia at 0°C gave the respective 3-chloro-2,6-diaryl-piperidin-4-ones 13–17. Cyclic ketones normally undergo Baeyer-Villeger oxidation (oxygen insertion reaction) to yield lactones upon treatment with peracids [Citation24,Citation25]. But, when 3-chloro-2,6-diaryl-piperidin-4-ones 13–17 were subjected to Baeyer-Villeger type of reaction by using m-chloroperbenzoic acid, 3-Chloro-1-hydroxy-2,6-diarylpiperidin-4-ones 18–22 resulted instead of lactones 23–27. The schematic representation and the analytical data of compounds 18–22 are given in and , respectively. Their proposed mechanism of formation is shown in . The structure of the newly synthesized compounds 18–22 was confirmed by melting point, elemental analysis, MS, FT-IR, one-dimensional NMR (1H & 13C) spectroscopic data.
Table I. Physical and analytical data of 3-chloro-1-hydroxy-2,6-diarylpiperidin-4-ones 18–22.
Antibacterial activity
Novel 3-Chloro-1-hydroxy-2,6-diarylpiperidin-4-ones 18–22 were tested for their antibacterial activity in vitro against S.aureus, β-H.streptococcus, V.cholerae, S.typhii, S.felxneri, E.coli, K.pneumonia and Pseudomonas. Ciprofloxacin was used as standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is shown in . All the synthesized 3-Chloro-1-hydroxy-2,6-diarylpiperidin-4-ones 18–22 exerted potent antibacterial activity in vitro against the tested gram-positive and gram-negative bacterial strains except E.coli. Moreover, compounds 21 and 22 exerted strong antibacterial activities against S.aureus, β-H.streptococcus, V.cholerae, S.typhii and Pseudomonas.
Table II. In vitro antibacterial activities (MIC) values for compounds 18–22.
Antifungal activity
The in vitro antifungal activity of the synthesized novel heterocyclic compounds, 18–22 was studied against the fungal strains viz., A.flavus, Mucor, Rhizopus and M.gypsuem. Fluconazole was used as a standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is shown in . Compounds 21 and 22 exerted strong antifungal activities against all the tested fungal strains. In addition, compound 20 was the most potent against Rhizopus.
Table III. In vitro antifungal activities (MIC) values for compounds 18–22.
Conclusion
A close inspection of the in vitro antibacterial and antifungal activity profile in differently electron donating (CH3 and OCH3) as well as electron withdrawing (Cl,F) functional group substituted phenyl rings of novel 3-Chloro-1-hydroxy-2,6-diarylpiperidin-4-ones 18–22 against the tested bacterial strains viz. S.aureus, β-H.streptococcus, V.cholerae, S.typhii, E.coli, K.pneumonia, Pseudomonas and the fungal strains viz., A.flavus, Mucor, Rhizopus and M.gypsuem respectively, provides a better structure activity relationship correlate, which may be summarized as follows: Results of this study show that the nature of substituents on the phenyl ring viz., methyl, methoxy, chloro, fluoro functions at the para positions of the aryl moieties are determinant for the nature and extent of the anti-bacterial activity of the synthesized compounds, which might have influences on their inhibiting mechanism of actions. Compounds 21 and 22, which contain chloro and fluoro moieties exerted strong antifungal activities against A.flavus, Mucor and M.gypsuem. The presence of electron donating methoxy functional moiety in compound 20 is most potent against Rhizopus. Further development of this group of 3-Chloro-1-hydroxy-2,6-diarylpiperidin-4-ones may lead to compounds with better pharmacological profile than standard drugs and serve as templates for the construction of better drugs to come to blow bacterial and fungal infections.
Acknowledgement
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore for recording spectra. Two of our authors namely J.Thanusu and V.Kanagarajan are highly thankful for Annamalai University authorities for providing financial support in the form of Research Fellowship.
References
- HI El-Subbagh, SM Abu-Zaid, MA Mahran, FA Badria, and AM Alofaid. (2000). Synthesis and biological evaluation of certain α, β-Unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J Med Chem 43:2915–2921.
- AA Watson, GWJ Fleet, N Asano, RJ Molyneux, and RJ Nugh. (2001). Polyhydroxylated alkaloids. Natural occurrence and therapeutic applications. Phytochemistry 56:265–295.
- CR Ganellin, and RG Spickett. (1965). Compounds affecting the central nervous system. 1,4-piperidones and related compounds. J Med Chem 8:619–625.
- RE Hagenbach, and H Gysin. (1952). Piperidones as potential antitumour agents. Experimentia 8:184–187.
- B Ileana, V Dobre, and I Nicluescu-Duvaz. (1985). Spin labeled nitrosoureas. Potential anticancer agents. J Prakt Chem 327:667–674.
- IG Mokio, AT Soldatenkov, VO Federov, EA Ageev, ND Sergeeva, S Lin, EE Stashenku, NS Prostakov, and EL Andreeva. (1989). Antimicrobial activity of aliphatic-aromatic ketones, β-ketols and α-glycols. Khim Farm Zh 23:421–427.
- JR Dimmock, and P Kumar. (1997). Anticancer and cytotoxic properties of mannich bases. Curr Med Chem 4:1–22.
- H Kubota, A Kakefuda, Y Okamoto, M Fujii, O Yamamoto, Y Yamgiwa, M Orita, K Ikeda, M Takenchi, T Shibanuma, and Y Fsomura. (1998). Synthesis of ( ± )-N-[2-(3,4-dichlorophenyl)-4-(spiro-substituted piperidin-1′-yl)butyl]-N-methylbenzamides and evaluation of NK[1]-NK[2] dual antagonistic activities. Chem Pharm Bull 46:1538–1544.
- M Krishnapillay, and MIF Mohamed. (1993). Preparation of some 3-chloro-substituted 2,6-diarylpiperidin-4-ones. J Indian Chem Soc 70:257–258.
- M Krishnapillay, and MIF Mohamed. (1997). Conformational studies on some 3-chloro-substituted 2,6-diarylpiperidin-4-ones by 1H NMR spectra. Indian J Chem 36B:50–53.
- JG Hardman, and LE Limbad. (1996). The pharmacological basis of therapeutics. IX ed. 1279. New York: McGraw Hill
- JG Hardman, and LE Limbad. (1996). The pharmacological basis of therapeutics. IX ed. 1187. New York: McGraw Hill
- JJ Tufariello, and EL George. (1980). Functionalized nitrones. A highly stereoselective and regioselective synthesis of dl-retronecine. J Amer Chem Soc 102:373.
- J Davies. (1996). Bacteria on the rampage. Nature 383:219.
- DTW Chu, JJ Plattner, and L Katz. (1996). New directions in antibacterial research. J Med Chem 39:3853.
- RV SperaJr, and BF Farber. (1994). Multidrug-resistant Enterococcus faecium. An untreatable nosocomial pathogen. Drugs 48:678–688.
- FC Odds. (1993). Resistance of yeasts to azole-derivative antifungals. J Antimicrob Chemother 31:463–471.
- CA Hitchock. (1993). Resistance of Candida albicans to azole antifungal agents. Biochem Soc Trans 21:1039–1047.
- EM Johnson, DW Warnock, J Luker, and SRJ Porter. (1995). Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. Antimicrob Chemother 35:103–114.
- JH Rex, MG Rinaldi, and MA Pfaller. (1995). Resistance of Candida species to flucanazole. Antimicrob Agents Chemother 39:1–8.
- M Gopalakrishnan, P Sureshkumar, J Thanusu, V Kanagarajan, R Govindaraju, and G Jayasri. (2007). A convenient ‘one-pot’ synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J Enz Inhib Med Chem 22 (6):709–715.
- M Gopalakrishnan, P Sureshkumar, J Thanusu, C Prabhu, and V Kanagarajan. (2007). One-pot conversion of piperidine-4-ones to [1,4]-diazepan-5-ones under microwave irradiation using silica gel supported NaHSO4 catalyst. J Chem Res 2:80.
- M Gopalakrishnan, P Sureshkumar, J Thanusu, and V Kanagarajan. (2007). Design, synthesis, characterization, antibacterial and antifungal activities of novel class of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-selenadiazoles. J Enz Inhib Med Chem (in press): DOI:10.1080/14756360701611498
- M Hudlicky . Oxidations in organic chemistry. Washington: American Chemical Society; (1990). p 186.
- GR Krow. (1981). Oxygen insertion reactions of bridged bicyclic ketones. Tetrahedron 37:2697–2724.
- MH Dhar, MM Dhar, BN Dhawan, BN Mehrotra, and C Ray. (1968). Screening of Indian plants biological activity. Part I. Indian J Exp Biol 6:232–247.