Abstract
Adenosine deaminase (ADA), an enzyme involved in purine metabolism, catalyzes the hydrolytic breakdown of adenosine into inosine and free ammonia. ADA regulation has been targeted as a potential therapeutic agent for viral infections and lymphoproliferative disorders. In this study, we isolated a novel ADA inhibitor from a culture of Bacillus sp. J-89, and evaluated its anti-proliferative activity on human cancer cell lines. The ADA inhibitor was deduced as a 2-N-methyl-2,4-diazacycloheptanone by analyses of UV, IR, EI-MASS, 1H-NMR, 13C-1H NMR, and 13C-NMR spectroscopy, and was designated IADA-7. IADA-7 was shown to inhibit purified mammalian and Actinomyces ADA. IADA-7 also inhibited the proliferation of both Jurkat T cells (IC50 = 15 μg/mL) and J 82 (human transitional-cell carcinoma, bladder) cells (IC50 = 25 μg/mL). In Jurkat T cells, apoptosis with 15 μg/mL IADA-7 for 24 and 48 hours was 9 and 13%, respectively. These results suggest that IADA-7 can inhibit ADA activity in multiple species and that it may represent a good candidate as an anti-cancer therapeutic agent due to its demonstrated anti-proliferative activity on cancer cells.
| Abbreviations | ||
| ADA, | = | adenosine deaminase |
| EI-MASS, | = | Electron Ionization Mass |
| HETCOR, | = | Heteronuclear Correlation |
| IR, | = | Infra-Red |
| NMR, | = | Nuclear Magnetic Resonance |
Introduction
Adenosine deaminase (ADA, adenosine aminohydrolase, EC 3.5.4.4), an enzyme involved in purine metabolism, catalyzes the irreversible deamination of adenosine and 2′-deoxyadenosine to inosine and 2′-deoxyinosine, respectively. Inosine and 2′-deoxyinosine are then further degraded to uric acid, or returned to the pool of purine [Citation1]. This enzyme is found ubiquitously in microorganisms, plants, invertebrates, vertebrates, and mammals, including humans, and its amino acid sequence is highly conserved from bacteria to humans Citation2–6.
ADA plays an important role in various physiological functions, including the differentiation and maturation of the lymphoid system. The critical role of ADA activity in T cell development has been demonstrated by the fact that the genetic deficiency of the enzyme in humans is associated with severe combined immunodeficiency disease (SCID) [Citation7,Citation8]. Most evidence suggests that ADA deficiency induces the abnormal accumulation of ADA substrates, which is detrimental to lymphocyte development and survival [Citation9]. Due to the important role of ADA in cellular proliferation and differentiation, ADA inhibitors have been considered to be highly promising candidates for antibiotics, anti-cancer drugs, and anti-viral drugs for hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections Citation10–16.
Over the last 50 years, many types of ADA inhibitors have been isolated from microorganisms. Examples of these include coformycin from S. kaniharaensis SF-557 [Citation17], pentostatin from S. antibioticus [Citation18], and cordycepsin from A. nidulans Y-176-2 [Citation19]. Alcohols derived from adenine nucleosides such as 9-α-D-mannopyranosyladenine, 9-ß-D-xylopyranosyladenine, and 9-ß-L-fucopyranosyladenine have also been found to inhibit [Citation20,Citation21]. However, most of these ADA inhibitors are also extremely toxic to normal human cells, and therefore have limitations in its clinical applications. Currently, only one ADA inhibitor, pentostatin, is in use for the treatment of leukemia [Citation22]. Therefore, it is of great importance to discover novel safe and effective ADA inhibitors for clinical applications. In this study, we identified and characterized a novel ADA inhibitor isolated from a soil bacterium.
Materials and methods
Preparation of ADA
For analysis of ADA inhibition, we prepared ADA from a bovine pancreas (Sigma) and from a Nocardioides sp. The bovine pancreatic ADA was dissolved in 0.05 M potassium phosphate buffer, pH 7.0 and diluted with the same buffer for the enzyme assay. The microorganism-derived enzyme was prepared by culturing Nocardioides sp. J-326TK [Citation23] and harvesting the culture supernatant.
Isolation of a bacterial strain producing an ADA inhibitor
We reported previously Bacillus sp. J-89, an ADA inhibitor producing bacterial strain [Citation24]. Briefly, to isolate a bacterial strain producing an ADA inhibitor, a soil sample collected from an area of Pusan was air-dried in darkness for 2 to 3 days. The dried soil sample (0.5 g) was suspended in 5 mL of distilled water and then allowed to settle for 10 min, and the supernatant taken from the suspension was plated onto Medium A (1% soluble starch, 3.7 mM KH2PO4, 9.3 mM NH4Cl, and 1.7% agar) followed by incubation at 30°C until colony formation. To determine whether the isolated bacterial colonies could synthesize ADA inhibitors, they were inoculated in Medium B (1% glucose, 0.2% yeast extract, 0.2% meat extract, 0.2% peptone, 0.5 mM MgCl2, and 3.7 mM KH2PO4) and grown at 30°C for 24 h in a water bath with shaking. After culturing, each culture supernatant was used for ADA activity assay to screen a bacterial strain producing ADA inhibitors and a bacterial clone, of which culture supernatant had ADA inhibitory activity, was isolated.
ADA assay
ADA assay was carried out according to the method described previously [Citation25]. Briefly, the various concentrations of ADA were combined with 5 μM adenosine (1 mL final volume) in the presence or absence of ADA inhibitor at 37°C for 30 min in 0.05 M potassium phosphate buffer, pH 7.0. The reaction was stopped by heat inactivation of the enzyme at 100°C for 4 min, then diluted 100-fold to monitor absorbance at 265 nm using a spectrophotometer (Beckman, USA). One unit of ADA activity was defined as the amount of enzyme that produced 1 μmole of inosine per hour under the above described condition.
Measurement of inhibitory activity of IADA-7
The inhibitory activity of the chemical compounds was measured by quantitating the rate of inhibition of the ADA deamination reaction. The inhibition rate was calculated using the difference between enzyme activity in the presence or absence of inhibitor. The amount of enzyme for each assay was about 17 ∼ 26 units/mL, and the concentration of test compounds was adjusted to inhibit up to 70% of ADA activity. One unit was defined as the inhibitory activity of 1 mL of sample that showed an inhibition rate of 50%. The inhibition rate and inhibitory activity were calculated as follows:
Inhibition rate = (enzyme activity-sample activity)/enzyme activity × 100 (%)
Inhibitory activity = inhibition rate/50 × dilution rate (units/mL)
Purification and structure determination of IADA-7
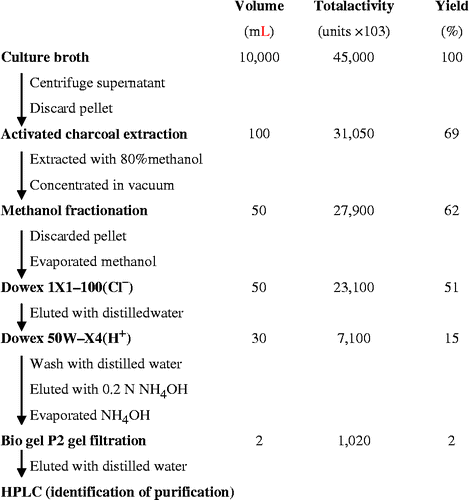
Bacillus sp. producing the ADA inhibitor was cultured in Medium B, and the ADA inhibitor was purified as shown in . Briefly, the 10 liter culture supernatant was separated by centrifugation, treated with 50 g activated charcoal (Sigma), and then extracted with 80% methanol. The extract was concentrated to 500 mL under reduced pressure, mixed with 500 mL of methanol, and then stored at − 20°C for 3 days. After methanol fractionation, the supernatant containing ADA inhibitory activity was concentrated under a vacuum, and then applied to a Dowex 1X1-100 (Cl− ) column (Sigma). ADA inhibitory activity was observed in the unbound fraction, and this fraction was applied to a Dowex 50W-X4 (H+) column, washed, and then eluted with 0.2 N NH4OH. The fractions containing ADA inhibitory activity were pooled and then further purified by Bio-gel P2 (Bio-Rad) gel filtration. After concentrating the fractions containing ADA inhibitory activity, the purified compound was dissolved in DMSO and stored at − 80°C. The chemical structure of the purified ADA inhibitor was determined as described in the Results.
Cytotoxicity assay
Cytotoxicity of the ADA inhibitor was measured according to Mossmann's method using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) with slight modifications [Citation26]. Human transitional-cell carcinoma J82 cells derived from the bladder and human Jurkat T cells were maintained in RPMI 1640 medium (Sigma) supplemented with 10% fetal calf serum (Gibco-BRL). Cells were plated at 1 × 105 cells/well in 96-well plates, and treated with or without IADA-7 at concentrations ranging from 1 to 50 μg/mL. After incubation for 44 h, 50 μL of MTT solution (Sigma) were added to the wells followed by incubation for another 4 h. The plates were then centrifuged, and the supernatant was removed. A 150 μL aliquot of DMSO was added to each well, and the absorbance was measured at 540 nm using an ELISA plate reader.
Flow cytometry analysis for apoptosis
After treatment with 15 μg/mL purified IADA-7, Jurkat T cells were prepared as single cell suspension at a concentration of 1 × 105 cells/0.1mL in PBS, following add 5 μL of Propidium Iodide Staining Solution (Becton Dickinson), according to the supplier's instructions. Briefly, gently vortex the cells and incubate for 15 min at RT (25°C), following add 400 μL of 1 × binding buffer to each tube. Findings characterizing flow for 1 × 105 cells were analyzed with CellQuest software (Becton Dickinson).
Results and discussion
Purification and structural identification of IADA-7
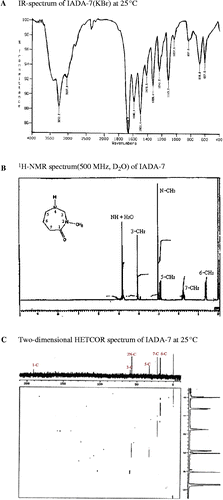
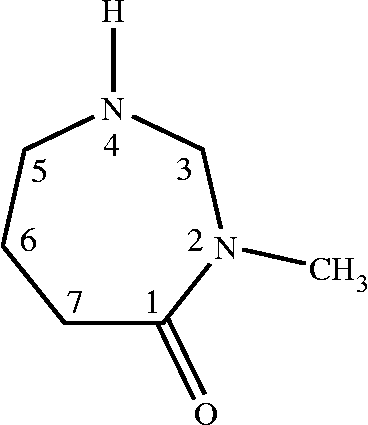
From the culture supernatant of Bacillus sp. IADA-7, adenosine deaminase inhibitor was sequentially purified as described in . The purified ADA inhibitor was observed as a single peak in high performance liquid chromatography (HPLC) analysis (data not shown). To determine the molecular structure of this compound, we collected and analyzed spectral data from infra-red (IR), proton nuclear magnetic resonance (1H-NMR), carbon nuclear magnetic resonance (13C-NMR), 13C-1H heteronuclear correlation (HETCOR), electron ionization mass (EI-Mass), and ultraviolet (UV) spectroscopies. The IR spectrum was obtained first to identify the functional groups in the molecule. Two significant bands at 3253 and 1670 cm− 1 indicated the presence of amine (NH) and amide carbonyl (C = O) groups (), respectively. The 1H-NMR and 13C-NMR spectra were particularly informative, as they allowed us to determine the number and type of protons and carbons in the molecule. The 1H-NMR spectrum (D2O, 500 MHz) was obtained at room temperature in D2O solvent with TMS as an internal standard, and it showed six resonance lines at 0.62 (6-CH2), 1.78 (7-CH2), 2.90 (5-CH2), 3.08 (N–CH3), 4.09 (3-CH2), and 4.85 (NH) ppm in the aliphatic region, and their chemical shifts, the splitting patterns and integration ratios of the peaks are in good agreement with the expected data of the proposed molecular structure (). However, the proton of the NH group at 4.85 ppm did not give the exact integration ratio, but also the peak of amine proton could not be detected by overlapping with the peaks of water protons, because the proton of the NH group was rapidly exchanged with the deuterium of D2O solvent to give only a strong water peak. The 13C-NMR spectrum (D2O, 125.65 Hz) showed six distinct resonance lines at 191, 59.0, 57.5, 32.6, 22.3, and 18.0 ppm. The signal at 191 ppm was easily assigned as the peak of the C = O group carbon. However, for explicit assignments of five remaining carbon signals in the aliphatic region, we applied two-dimensional NMR techniques to obtain HETCOR spectrum (). Each spot on the HETCOR plot allows us to clearly identify the relations between proton and carbon peaks. The carbon peak at 18.0 ppm and the proton multiplet at 0.62 ppm correspond to the methylene group at the 6-position; the carbon peak 22.3 ppm and the proton multiplet at 1.78 ppm correspond to the methylene group at the 7-position and the carbon peak at 32.6 ppm, and the proton multiplet at 2.90 ppm correspond to the methylene group at the 5-position. The other carbon peak of the methylene group at the 3-position is deshielded by the two neighboring nitrogen atoms. Therefore, the spot on the HETCOR plot for this methylene group appears at 59.0 ppm on the carbon axis and 4.09 ppm on the proton axis. The remaining spot for the methyl group (2N–CH3) on the HETCOR plot appears at 57.5 ppm on the carbon axis and 3.08 ppm on the proton axis. Based on the above results, we could clearly determine the molecular structure of IADA-7 as described in . The EI-Mass spectrum of this compound gave a direct evidence for this molecular structure, showing a molecular ion peak at m/z = 128 (M+), a base peak at m/z = 113 (M+–CH3), and several fragmentation peaks at m/z = 97, 84, 69, and 57. The UV absorption spectrum of this compound showed two absorption bands at 204 and 239 nm, which correspond to the p–p* and n–p* transition bands of the carbonyl group, respectively. The absorption bands are in good agreement with typical lactam bands. Based on our conclusive structural analysis, we named this novel compound according to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature system: 2-N-methyl-2, 4-diazacycloheptanone.
Cytotoxic effect of IADA-7 on human cancer cells
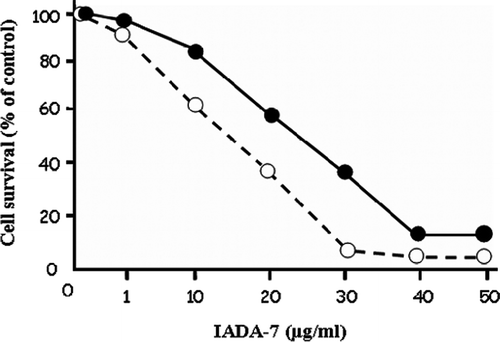
Purified inhibitor was shown to inhibit bovine pancreas ADA and Actinomyces ADA (data not shown). Since it has been well known that ADA inhibitor is a good candidate for use as an anticancer drug [15,20,22,24,29], the cytotoxic effect of IADA-7 on Jurkat T cells and J 82 cells was investigated using an MTT assay. When both cells lines were treated with an increasing concentration of IADA-7 (1 to 50 μg/mL), cytotoxicity to both cell types was observed in a dose-dependent manner (). The IC50 value of IADA-7 on Jurkat T cells and J 82 cells was 15 and 25 μg/mL, respectively.
Figure 4. Cytotoxic effect of IADA-7 on human Jurkat T cells (- - -○- - -) and human bladder carcinoma J 82 cells (•). Both cell lines were treated with the indicated concentration of IADA-7, and its cytotoxic effect was assessed via an MTT assay, as described in the Materials and Methods.
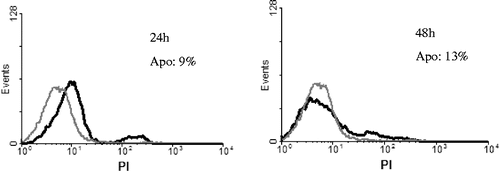
To quantitate whether the cause of anti-cancer effect in vivo was due to the IADA-7 induced apoptosis. Jurkat T cells were treated with 15 μg/mL of IADA-7 for 24 and 48 h. The apoptotic cells were detected by flow cytometry (). A typical apoptotic cells treated with IADA-7 for 24 and 48 h were 9 and 13%, respectively. This result suggests that the exposure in IADA-7-mediated apoptotic cells was in a time-dependent manner.
Figure 5. Effect of purified IADA-7 on Jurkat T cells analysed by flow cytometry. Cells were treated with IADA-7 (15 μg/mL) for 0–48 h and assessed by propidium iodide (PI) staining, as described in Materials and Methods. Grey line represents control cells, and black line represents 24 h, 48 h sample cells, respectively. Apo: apoptotic percentage.
In this study, we have isolated a novel ADA inhibitor, IADA-7, from Bacillus sp. and characterized its structural and chemical properties. IADA-7 contains a unique ring structure that is not found in conventional ADA inhibitors or purine analogs. It is noteworthy that the IC50 value of coformycin, a representative conventional ADA inhibitor, was 5 μg/mL on Jurkat T cells (data not shown). However, the IC50 value of IADA-7 was 15 μg/mL, which is much lower than the conventional ADA inhibitors tested on the same cells. This large difference in inhibitory potency may be due to the intrinsic differences in chemical structures. Future studies in our laboratory include the comparison of its cytotoxic activity with that of other ADA inhibitors.
The ADA enzyme plays a critical role in cell proliferation and survival, since it is involved in the biosynthesis and metabolism of purine nucleotides. ADA is most abundant in lymphoid tissues, and this may explain why a deficiency in this enzyme causes SCID in humans [Citation10,Citation21]. By virtue of their central role in the proliferation of lymphoid cells, specific inhibitors of ADA have been proposed as candidates to treat tumors and other cellular proliferative diseases. Extensive screening for ADA inhibitors has resulted in the identification of a number of inhibitory compounds predominantly from Actinomyces sp. To our knowledge, this is the first report of an ADA inhibitor produced by Bacillus strain.
As previously noted, ADA has emerged as an attractive target for anti-cancer drug development due to its anti-proliferative activity. For example, pentostatin displays a striking anti-tumor activity on several lymphoid cancers, and is currently in use for the treatment of lymphoid malignancies [Citation25]. Similarly, the new ADA inhibitor IADA-7 showed a significant cytotoxic effect on human Jurkat T cells (Figures , ). The anti-proliferative effect of IADA-7 implies that IADA-7, and possibly its analogs, could represent good candidates for lead compounds in the development of drugs for specific cancers. Therefore, it is of great interest to optimize the structure of IADA-7 to increase its affinity for ADA and its cytotoxic effect on cancer cells. Our laboratory is currently investigating the exact properties of IADA-7, including ADA binding, inhibitory mechanisms, and binding specificity to improve upon the drug-like properties of IADA-7.
Acknowledgements
This work was supported by the Korea Health 21 R&D project, Ministry of Health and Welfare, Korea (A030001).
References
- G Cristalli, S Costanzi, C Lambertucci, G Lupidi, S Vittori, R Volpini, and E Camaioni. (2001). Adenosine deaminase: Functional implications and different classes of inhibitors. Med Res Rev 21:105–128.
- TG Brady, and VJ Hegarty. (1966). An investigation of plant seeds for adenosine deaminase. Nature 209:1027–1028.
- ZY Chang, P Nygaard, AC Chinault, and RE Kellems. (1991). Deduced amino acid sequence of Escherichia coli adenosine deaminase reveals evolutionarily conserved amino acid residues: Implications for catalytic function. Biochemistry 30:2273–2280.
- PE Daddona. (1981). Human adenosine deaminase. Properties and turnover in cultured T and B lymphoblasts. J Biol Chem 256:12496–12501.
- MA Kelly, MM Vestling, CM Murphy, S Hua, T Sumpter, and C Fenselau. (1996). Primary structure of bovine adenosine deaminase. J Pharm Biomed Anal 14:1513–1519.
- D Shi, JH Winston, MR Blackburn, SK Datta, G Hanten, and RE Kellems. (1997). Diverse genetic regulatory motifs required for murine adenosine deaminase gene expression in the placenta. J Biol Chem 272:2334–2341.
- F Foss. (2003). Overview of cutaneous T-cell lymphoma: Prognostic factors and novel therapeutic approaches. Leuk Lymphoma 44 (Suppl 3):S55–S61.
- GC Mills, FC Schmalstieg, KB Trimmer, AS Goldman, and RM Goldblum. (1976). Purine metabolism in adenosine deaminase deficiency. Proc Natl Acad Sci U S A 73:2867–2871.
- R Hirschhorn. (1993). Overview of biochemical abnormalities and molecular genetics of adenosine deaminase deficiency. Pediatr Res 33:S35–S41.
- R Hirschhorn, F Martiniuk, and FS Rosen. (1978). Adenosine deaminase activity in normal tissues and tissues from a child with severe combined immunodeficiency and adenosine deaminase deficiency. Clin Immunol Immunopathol 9:287–292.
- K Borroto-Esoda, F Myrick, J Feng, J Jeffrey, and P Furman. (2004). In vitro combination of amdoxovir and the inosine monophosphate dehydrogenase inhibitors mycophenolic acid and ribavirin demonstrates potent activity against wild-type and drug-resistant variants of human immunodeficiency virus type 1. Antimicrob Agents Chemother 48:4387–4394.
- SM Daluge, SS Good, MB Faletto, WH Miller, MH St Clair, LR Boone, M Tisdale, NR Parry, JE Reardon, RE Dornsife, DR Averett, and TA Krenitsky. (1997). 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother 41:1082–1093.
- E Fernandez, L Rodrigo, S Riestra, S Carcia, F Gutierrez, and G Ocio. (2000). Adenosine deaminase isoenzymes and neopterin in liver cirrhosis. J Clin Gastroenterol 30:181–186.
- HP Guan, MB Ksebati, YC Cheng, JC Drach, ER Kern, and J Zemlicka. (2000). Spiropentane mimics of nucleosides: Analogues of 2′-deoxyadenosine and 2′-deoxyguanosine. Synthesis of all stereoisomers, isomeric assignment, and biological activity. J Org Chem 65:1280–1290.
- G Iliakis, and FQ Ngo. (1985). Effects of adenosine deaminase inhibitor 2′-deoxycoformycin on the repair and expression of potentially lethal damage sensitive to beta-araA. Radiat Environ Biophys 24:81–88.
- JB Tunac, and M Underhill. (1985). 2′-Chloropentostatin: Discovery, fermentation and biological activity. J Antibiot (Tokyo) 38:1344–1349.
- T Sawa, Y Fukagawa, I Homma, T Takeuchi, and H Umezawa. (1967). Mode of inhibition of coformycin on adenosine deaminase. J Antibiot (Tokyo) 20:227–231.
- PW Woo, and DC Baker. (1982). Inhibitors of adenosine deaminase. Studies in combining high-affinity enzyme-binding structural units. erythro-1,6-Dihydro-6-(hydroxymethyl)-9-(2-hydroxy-3-nonyl)purine and erythro-9-(2-hydroxy-3-nonyl)purine. J Med Chem 25:603–605.
- RH Adamson, DW Zaharevitz, and DG Johns. (1977). Enhancement of the biological activity of adenosine analogs by the adenosine deaminase inhibitor 2′-deoxycoformycin. Pharmacology 15:84–89.
- RP Agarwal, T Spector, and RE ParksJr. (1977). Tight-binding inhibitors–IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol 26:359–367.
- AJ Grant, and LM Lerner. (1979). Hexofuranosyladenine nucleosides as substrates and inhibitors of calf intestinal adenosine deaminase. J Med Chem 22:1016–1018.
- MM Oken, S Lee, NE Kay, W Knospe, and PA Cassileth. (2004). Pentostatin, chlorambucil and prednisone therapy for B-chronic lymphocytic leukemia: A phase I/II study by the Eastern Cooperative Oncology Group study E1488. Leuk Lymphoma 45:79–84.
- HK Jun, TS Kim, and Y Yeeh. (1994). Purification and characterization of an extracellular adenosine deaminase from Nocardioides sp. J-326TK. Biotechnol Appl Biochem 20 (Pt 2):265–277.
- YK Shin, YH Park, JD Lee, and HK Jun. (1997). Identification of adenosine deaminase inhibitor-producing bacterium isolated from soil. J Microbiol Biotechnol 7:32–36.
- GL Tritsch. (1983). Validity of the continuous spectrophotometric assay of Kalckar for adenosine deaminase activity. Anal Biochem 129:207–209.
- T Mosmann. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63.
- H Tanaka, T Kawakami, ZB Yang, K Komiyama, and S Omura. (1989). Potentiation of cytotoxicity and antitumor activity of adenosine analogs by the adenosine deaminase inhibitor adecypenol. J Antibiot (Tokyo) 42:1722–1724.