Abstract
Complexes of the type [Al(HL)(OH)Cl2], [M(HL)(OH)2Cl] and [M′(HL)(L′)(OH)Cl], where HL = 5-iodouracil; HL′ = histidine; M = Cr(III), Fe(III) and M′ = Al(III), Cr(III), Fe(III), were synthesized and characterized. The complexes are polymeric showing high decomposition points and are insoluble in water and common organic solvents. The μeff values, electronic spectral bands and ESR spectra suggest a polymeric 6-coordinate spin-free octahedral stereochemistry for the Cr(III) and Fe(III) complexes. 5-Iodouracil acts as a monodentate ligand coordinating to the metal ion through the O atom of C(4) = O while histidine through the O atom of –COO− and the N atom of –NH2 group. In vivo antitumour effect of 5-iodouracil and its complexes was examined on C3H /He mice against P815 murine mastocytoma. As evident from their T/C values, Cr(III) and Fe(III) complexes display significant and higher antitumour activity compared to the 5-iodouracil ligand. The in vitro results of the complexes on the same cells indicate that Cr(III) and Fe(III) complexes show higher inhibition on 3H-thymidine and 3H-uridine incorporation in DNA and RNA replication, respectively, at a dose of 5 μg/mL.
Introduction
The role of several drugs in relation to their metal binding has been established. The antitumour activity of platinum(II) complexes with nucleosides and their bases have been investigated extensively [Citation1–4]. It is accepted that the antitumour activity is due to inhibition of DNA synthesis in cancer cells [Citation5]. Aluminium(III) has been shown to bind with the chelating ligands in a simple human blood plasma model [Citation6]. It has been established that the liability of chromium(III) complexes with distorted octahedral geometry is the possible factor for its active role in biological systems [Citation7]. Iron is quite essential to oxidative metabolism of all body cells as well as in enzymatic functions. Iron deficiency produces many structural and functional abnormalities [Citation8].
It has been suggested that metal ions play a significant role in the maintenance of the configuration of the nucleic acid molecule possibly linking nucleic acid bases through covalent bonds. 5-Iodouracil is similar to nucleic acid bases and has some antitumour properties. It has lethal and mutagenic effects on bacteriophage T4 [Citation9]. The lethal effect of 5-iodouracil is a consequence of its incorporation into DNA. It has a lethal effect on the adult Drosophila melangaster also, by inhibiting egg-laying [Citation10].
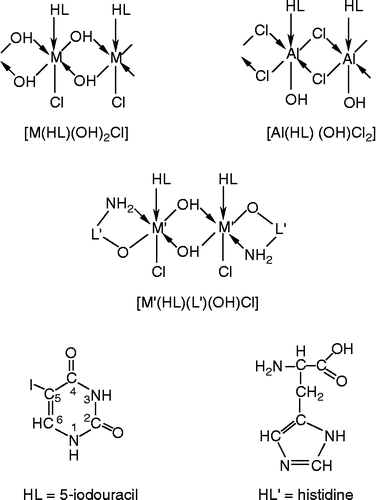
Metal ions in biological systems promote the interaction of proteins and nucleic acids through the formation of ternary complexes. The formation of nucleic acid-enzyme-transition metal ternary complexes during DNA replication and RNA synthesis are known [Citation11]. The study of such ternary complexes involving 5-iodouracil and amino acid like L-histidine may provide models for the more complicated metal-protein-nucleic acid interaction. A few bivalent transition metal complexes with uracil derivatives and its mixed ligand complexes have been reported [Citation12–14]. In continuation of our earlier work on metal(III) complexes of uracil derivatives [Citation15] and our interest in the biological applications of such complexes, we have synthesized and characterized a number of aluminium(III), chromium(III) and iron(III) complexes with 5-iodouracil and 5-iodouracil–histidine ligands () and investigated their antitumour properties. The results are discussed in this paper.
Experimental
Materials
All chemicals used were of AnalaR or equivalent grade. The metal chlorides employed were of E. Merck grade. 5-Iodouracil was purchased from Ega Chemie Co., Germany and L-histidine from Sisco Research Laboratory (SRL), India. 3H-thymidine and 3H-uridine used for antitumour activity was obtained from the Bhabha Atomic Research Centre, Mumbai. The mice used for the antitumour activity studies were obtained from Tata Institute of Fundamental Research, Mumbai. The P815 (murine mastocytoma) tumor cells was obtained from Tata Memorial Cancer Research Institute, Mumbai and was maintained in Prof. Ajit Sodhi's Research Laboratory, School of Biotechnology, Banaras Hindu University, Varanasi.
Preparation of the complexes
5-Iodouracil complexes
A quantity of 1 mmol of 5-iodouracil (0.2380 g) was dissolved in a mixture of 35 mL ethanol and 15 mL triethyl orthoformate. The solvent triethyl orthoformate was used to increase the solubility of 5-iodouracil ligand. To this solution was added 1mmol of AlCl3.6H2O (0.2415 g) in 10 mL hot ethanol and refluxed for 2-3 h at the boiling point. A clear solution was thus obtained. From this solution, the metal complex was precipitated by raising the pH to between 5 and 6 by adding drop wise 0.2 N NaOH. The precipitate was digested, filtered, washed with ethanol and ether successively and dried in an oven at 50°C. Fe(III) and Cr(III) complexes were prepared by taking 1 mmol of FeCl3.6H2O (0.2705 g) and CrCl3.6H2O (0.2665 g), respectively, in 10 mL hot ethanol under similar experimental conditions.
5-Iodouracil-histidine complexes
Using the same quantities of 5-iodouracil and metal salts and conditions of precipitation maintained as above, 1 mmol (0.1550 g) L-histidine, dissolved in 10 mL of hot distilled water, was added in each case after the precipitation of the complex. On reacting with L-histidine the precipitate was again dissolved. The resultant solution was concentrated over a water bath to 50% of its volume. The mixed-ligand complexes were precipitated by adjusting the pH up to 6 by adding few drops of 0.2 N NaOH. The precipitates were filtered and washed with ethanol and finally with ether and dried in an oven at 50°C.
Analysis and instrumentation
The metal contents of the complexes were determined after dissolving the complexes in very dilute HNO3 and titrating the excess 0.01 M EDTA at pH 5 by following the standard procedures [Citation16]. Analyses of C, H and N were carried out micro analytically on a Perkin-Elmer 240C model micro analyzer.
Room temperature magnetic susceptibilities of the complexes were determined with a Faraday type balance (Cahn magnetic susceptibility apparatus) using [CoHg(SCN)4] as calibrant and correcting the experimental values for diamagnetism [Citation17]. Electronic spectra of the complexes were recorded in Nujol mull on a Shimadzu 160A UV-visible recording spectrophotometer. Infrared spectra of the ligands and their complexes were recorded in Nujol mull on a Perkin Elmer 783 infrared spectrophotometer. X-band ESR spectra of the Iron(III)-5-iodouracil-histidine complex were recorded on a JESME-3X type spectrometer of powdered sample at room temperature and liquid nitrogen temperature using diphenyl picryl hydrazyl (DPPH) as g marker (g = 2.00238).
Antitumour activity evaluation
Animal experimentation was in accord with protocols laid down by the Department of Shalya and Centre of Experimental Medicine and Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
In vivo testing
C3H/He mice of either sex, about 6 - 8 weeks old and average body weight of 20 g, were used for in vivo antitumour activity tests against P815 (murine mastocytoma). Six animals were used for each set of experiment. An intraperitoneal injection of 2 × 105 tumour cells in phosphate buffered saline solution was given to each mouse at weekly intervals. Fine suspension of the test compounds, prepared in 0.89% saline solution (NaCl), were injected once a week after two days after the tumour transplantation at doses of 12.5 and 25 mg/kg body weight of mouse. The same volume of sterile saline solution was injected in the control set.
The therapeutic effectiveness of each compound against tumour bearing mice was assessed from the ratios of the mean survival time (in days) of the treated animals and control, and the T/C percentage was calculated as
*Excluding tumour free survivor
In vitro testing
A P815 murine mastocytoma tumour cell suspension (1 × 106 cells/mL) was prepared in a medium containing the tissue culture of RMPI 1640 supplemented with antibiotics, penicillin, streptomycin and 10% heat inactivated fetal calf serum. For the determination of the effects of various compounds on DNA replication, duplicate culture plates (NUNC, Denmark) containing 96 wells in each plate were taken and 2 × 106 tumour cells were added to each cell well. The test compounds at different doses (1, 5 and 10 μg/mL) were added in one set of culture plates while the other set was without test compound. After 24 h of incubation in a CO2 incubator at 37°C, the cells were washed thrice with a RMPI 1640 culture medium by centrifugation for 10 minutes. The cell pellets were resuspended in 0.2 mL of a medium of RMPI 1640+ antibiotics +10% heat inactivated fetal calf serum containing 0.5 μCi/mL 3H-thymidine for 18 h of reincubation. Again, cell pellets were centrifuged and the supernatant liquid was discarded, washed thrice with a balanced salt solution (phosphate buffered normal saline solution). The cell pellets were digested with 0.5% sodium dodecyl sulphate (SDS) and the effluent was counted for radioactivity in a LKB β-liquid scintillation counter. Since the test compounds inhibit the incorporation of 3H-thymidine in DNA of the tumour cells, the percentage inhibition was calculated as
where CPM = counts per minute of radioactivity of 3H-thymidine.
For the determination of the effect of the compounds on RNA synthesis, 3H-uridine (0.5 μCi/mL) was used in place of 3H-thymidine and the other procedures were the same as discussed above.
Results and discussion
It appears from the analytical data of the complexes () that the 5-iodouracil complexes display 1:1 (M:L) stoichiometry and the mixed ligand complexes exhibit 1:1:1 (M:L:L′) stoichiometry. The course of reactions is shown below:
Table I. Analytical and electronic spectral data of the complexes.
The Al(III) complexes are colourless while the Fe(III) and Cr(III) complexes are coloured. They are insoluble in water and other common organic solvents like ethanol, benzene, chloroform, carbon tetrachloride, acetone, acetonitrile, pyridine, ether, DMF and DMSO. The Al(III) complexes melt between 276–280°C while Fe(III) and Cr(III) complexes decompose without melting above 300°C.
Magnetic moments and electronic spectra
Both Cr(III) complexes show μeff values of 3.66 and 3.75 B.M., respectively, corresponding to three unpaired electrons, suggesting a high–spin octahedral stereochemistry [Citation18]. The Fe(III)-5–iodouracil and Fe(III)-5-iodouracil-histidine complexes exhibit μeff values of 5.36 and 5.26 B.M., respectively. Both of these values are slightly less than the corresponding value for 5 unpaired electrons (μeff = 5.94 B.M.) indicating magnetic exchange interaction due to polymerization.
The octahedral Cr(III) complexes are expected to show three spin allowed d-d transitions viz. 4A2g(F) → 4T2g (v1), → 4T1g(v2) and → 4T1g(P) (v3). In both Cr(III) complexes in this study only two bands are observed at 530, 535 nm (v1) and 345, 351 nm (v2), respectively. The v3 band, expected below 300 nm overlaps with the ligand bands or L → M charge transfer bands and is, m therefore, not assigned [Citation19]. A slight splitting in the above bands of the Cr(III) complexes is also observed which may partly be due to low–symmetry, hexa-coordinated configurations and partly due to the presence of different chromophores in the polymeric complexes.
The first two bands appearing in the Fe(III)-5-iodouracil and Fe(III)-5-iodouracil-histidine complexes at 512, 490 nm and 393, 389 nm, respectively, may be assigned to 6A1g 4T1g and 4T2g transition. The third band at 347 and 350 nm for the two complexes, respectively, may be due to the L → M charge transfer which obscures the low intensity d-d absorption bands.
ESR spectra
ESR spectra of the powdered sample of the Fe(III)–5-iodouracil–histidine complex at room temperature (300 K) and liquid nitrogen temperature (77 K) were obtained, but in each case there was simply a strong broad band with no evidence of fine structure. These results merely indicate strong dipolar coupling between the paramagnetic ions, as expected for a polymeric structure. However the fact that giso = 2.031 at RT and 2.035 at LNT suggests an octahedral environment around Fe(III) [Citation15].
IR spectra
5-Iodouracil complexes
Some important IR frequencies of 5-iodouracil and its complexes are given in . There are several absorption bands in the region 3500–3000 cm− 1 which may be attributed to –NH and –OH stretching modes. The v(O-H) band appearing at 3400, 3370 and 3406 cm− 1 in the Al(III), Cr(III) and Fe(III) complexes, respectively, indicates the presence of water molecule or O-H group [Citation20]. The absence of v(M-O) (aqua) bands in the lower region of the spectra of the complexes confirms the bonding of the O-H group. The medium-broad band at ca. 950 cm− 1 in the Cr(III) and Fe(III) complexes may be assigned as OH bridging, suggesting an OH bridged polymeric structure for the complexes.
Table II. Important IR spectral data of the 5-iodouracil complexes.
The v(C(4) = O) band occurring at 1657 cm− 1 in 5-iodouracil is shifted considerably towards lower frequency (ca. 20-40 cm− 1) in all the complexes, suggesting the coordination of the C(4) = O group to the metal. The v(C(2) = O), δ(N(1)-H), v(C-I) and δ(N(3)-H) bands appear at 1710, 1512, 1466 and 1430 cm− 1 in free 5-iodouracil. These bands either do not shift or show a slight shift in the metal complexes indicating that these groups are not taking part in coordination. The metal-oxygen stretching vibrations appear in the region 225–245 cm− 1 for six and at 276 cm− 1 for four-coordination [Citation15], the presence of characteristic bands in the region 245–250 cm− 1 strongly favours the coordination number six for all complexes. In the Al(III)-5-iodouracil complex, particularly, v(M-Cl) occurs at significantly lower wave numbers relative to the other complexes. This may be due to the presence of a bridging chloro ligand in the complex [Citation21].
5-Iodouracil-histidine complexes
In all the 5-iodouracil-histidine mixed ligand complexes, 5- iodouracil shows similar trends of bonding and shifting in the affected group frequencies as described earlier for the 5-iodouracil complexes. In addition, some new vibrations due to histidine coordination are observed in these complexes. The bands due to the C-N and C = N groups of the imidazole moiety of histidine do not shift in the spectra of the metal complexes (), suggesting non-involvement of imidazole moiety in the coordination. The v(COO− ) bands of histidine show a shift towards lower wave number in the complexes providing evidence that histidine is bonded to the metal ion through the carboxylic group [Citation22]. Histidine shows a band at 3360 cm− 1 due to v(N-H) of imidazole moiety remains unaffected in the metal complexes, suggesting that the N-H group of imidazole is not involved in bonding. Histidine also shows v(NH3+) at 3071 cm− 1, which disappears on coordination, suggesting coordination of histidine through the nitrogen of the amino group. This is further supported by the disappearance of δ(N-H) of –NH3+ at 1503 cm− 1 in histidine on complex formation.
Table III. Important IR spectral data of the 5-iodouracil-histidine complexes.
Based on the above discussions, the general structures in for the metal complexes are proposed.
Antitumour properties
In vivo effect
The in vivo effect of the ligand, 5-iodouracil, and its Al(III), Fe(III) and Cr(III) complexes in this study on P815 murine mastocytoma were evaluated on the basis of their percent T/C value. A T/C value of 115 indicates significant activity whereas, >125 indicates that the compound is very useful for testing on other tumour systems [Citation23]. At the dose of 25 mg/kg body weight, the compounds were cytotoxic and, therefore the results obtained at the dose of 12.5 mg/kg body weight were recorded. The experimental data () indicate that the 5-iodouracil itself shows significant antitumour activity (%T/C = 117) and the Cr(III) and Fe(III) complexes show greater antitumour activity than 5-iodouracil ligand. The Cr(III)-5-iodouracil complex in particular, shows the highest activity, with %T/C = 150, among all other complexes in the present study at the above dose. The Al(III) complexes show very low antitumour activity.
Table IV. In vivo antitumour activity.
In vitro effect
The data obtained for the inhibitory effect of DNA and RNA replication in vitro () of the compounds indicates that the activity increases at higher doses, but all the compounds were cytotoxic at the dose of 10 μg/mL. All complexes show maximum inhibition of DNA and RNA replication at a dose of 5 μg/mL. The Cr(III)-5-iodouracil complex, which showed the highest activity by the in vivo studies, again shows the maximum inhibition of DNA and RNA replication in vitro. The Fe(III) complexes also show significant in vitro antitumour activity while both Al(III) complexes were found to have poor inhibitory effect on DNA as well as RNA replication.
Table V. In vitro antitumour activity.
Acknowledgements
The authors thank the Head, Department of Chemistry, Banaras Hindu University for laboratory facilities, Prof. G. C. Prasad, Department of Shalya and Centre of Experimental Medicine and Surgery, Institute of Medical Sciences, B.H.U. for in vivo and Prof. Ajit Sodhi, School of Biotechnology, Banaras Hindu University for in vitro antitumour testing of the compounds.
References
- W Saenger. Principles of nucleic acid structure. Berlin: Springer-Verlag, (1983).
- B Lippert, G Raudaschl, CJL Lock, and P Pilon. (1984). Real model compounds for intrastand cross-linking of 2-guanine bases by cis-platine. Inorg Chim Acta 93:43.
- KJ Barnham, ZJ Guo, and PJ Sadler. (1996). Stabilization of monofunctional platinum-nucleotide adducts. J Chem Soc Dalton Trans 2867.
- J Arpalahti, KD Klika, R Sillanpaa, and R Kivekas. (1998). Dynamic processes in platinum(II)-adenosine complexes. Preparation, NMR spectroscopic characterization and crystal structure of isomeric Pt-II (diene)-adenosine complexes. J Chem Soc Dalton Trans 1397.
- W Jiazhu, H Jingshuo, H Liyiao, S Dashuang, and H Shangzhi. (1988). Antitumor activity of organotin compounds. Reaction, synthesis and structure of Et2SnCl2(phen) with 5-fluorouracil. Inorg Chim Acta 152:67.
- DJ Clevette, and C Orvig. (1990). Comparison of ligands of differing denticity and basicity for the invivo chelation of aluminium and gallium. Polyhedron 9:151.
- JC Chang, LE Gerdom, NC Baenziger, and HM Goff. (1983). Synthesis and molecular structure determination of carboxyl bound nicotinic acid (niacin) complexes of chromium(III). Inorg Chem 22:1739.
- AJ Leninger. Principles of biochemistry 2nd ed. New York: Worth Publishers Inc; (1984).
- DM Byrd, and WH Prusoff. (1975). Multiplicity reactivation of 5-iodouracil-substituted, nonviable bacteriophage T4TD8. Antimicrob Agents Chemother 8:558.
- MM Clynes, and EJ Duke. (1976). Developmental effects of inhibitors of nucleotide-metabolism in Drosophila. J Insect Physiol 22:1709.
- S Kasselouri, A Garoufis, and N Hadjiliadis. (1987). Pd(II) Pt(II) ternary complexes with nucleosides and amino acids. Inorg Chim Acta 135:L23.
- R Ghose. (1989). Complex formation of adenine–uracil base pair with some transition metal ions. Inorg Chim Acta 156:303.
- UP Singh, BN Singh, AK Ghose, RK Singh, and A Sodhi. (1991). Synthesis, characterization, and antitumor activity of 5-iodouracil complexes. J Inorg Biochem 44:277.
- UP Singh, R Ghose, AK Ghose, RK Singh, A Sodhi, and B Geeta. (1991). Antitumor activity studies of some synthesized transition metal complexes. Ind J Cancer Chemother 13:45.
- VP Singh, A Singh, KK Narang, and D Bhattacharya. (2004). 5-Bromouracil and 5-bromouracil-histidine complexes with metal(III) ions and their antitumour activity. Trans Met Chem 29:107.
- AI Vogel. Vogel's text book of quantitative chemical analysis 5th ed. Amsterdam: Longman; (1989).
- RL Dutta, and A Syamal. Elements of magnetochemistry 2nd ed. New Delhi: Affiliated East-West Press Pvt. Ltd; (1993).
- FA Cotton, G Wilkinson, CA Murillo, and M Bochmann. Advanced inorganic chemistry 6th ed., New York: John Wiley & Sons Inc; (2003).
- ABP Lever. Inorganic electronic spectroscopy 2nd ed. Amsterdam: Elsevier; Amsterdam, (1984).
- K Nakamoto. Infrared and Raman spectra of inorganic and coordination compounds. New York: Wiley Interscience; (1986).
- CM Mikulski, S Cocco, N Defranco, and NM Karayannis. (1983). Cromium(III) chloride complexes with purine and adenine. Inorg Chim Acta 80:L71.
- KK Narang, JP Pandey, and VP Singh. (1994). Synthesis, characterization and physicochemical studies of some copper(II) tetrathiocyanato dithallate(I) complexes with hydrazides and hydrazones. Polyhedron 13:529.
- S Agrawal, NK Singh, RC Aggarwal, A Sodhi, and P Tandon. (1986). Synthesis structure and antitumor activity of N-salicyloyl-N′-(2-furylthiocarbonyl) hydrazine and its copper(II) complexes. J Med Chem 29:199.