Abstract
A series of hydrazone and 3-nitrovinyl analogs of indole-3-carboxaldehydes and related compounds were synthesized and screened for antitubercular activity against Mycobacterium tuberculosis H37RV in BACTEC 12B medium using the Microplate Alamar Blue Assay (MABA). Several compounds showed inhibitory activity against M. tuberculosis in primary screening assays at a concentration of 6.25 μg/mL; subsequent dose-response studies indicated that the most active compounds, 3d, 3e & 8b, had IC50 values of 5.96, 5.4 & 1.6 μg/mL, respectively. These compounds represent potential leads for the further development of novel antitubercular agents.
Introduction
Tuberculosis is a contagious disease caused by Mycobacterium tuberculosis. Despite the ready availability of effective treatments, tuberculosis today still represents one of the major worldwide public health problems. After a long period in which this disease seemed to be declining, in the last two decades an unexpected return has been observed [Citation1]. The World Health Organization has recently estimated that every year about eight million new cases of tuberculosis occur, and up to three million individuals die from the disease [Citation2]. Isoniazid, pyraziniamide, ethambutol, rifampicin and streptomycin, drugs generally used in combination, are still the current drugs of choice in the therapy of tuberculosis [Citation3]. However, the protracted use of these molecules overtime represents the main cause in the outbreak of new resistant bacterial strains. Therefore, there is now an urgent need for the development of new antitubercular drugs.
Numerous recent reports in the literature provide evidence of a renewed interest in the search for new antitubercular agents. The discovery that the thiosemicarbazone analog of indole-3-carboxaldeyde is active against M. tuberculosis in mice [Citation4,Citation5] provided the stimulus for us to prepare a variety of structurally related compounds. A wide range of biological activities has been claimed for derivatives of indole-3-carboxaldeydes [Citation6–11]. Importantly, the isonicotinylhydrazone of 1-benzylindole-3-carboxaldehyde is claimed to have antitubercular activity [Citation12].
A variety of nitrovinylindoles derived from indole-3-carboxaldehydes have also been shown to possess antifungal [Citation13] and amoebicidal activity [Citation14]. However, there are no reports on the antitubercular activity of nitrovinylindoles. Thus, several nitrovinylindoles have also been investigated for antitubercular activity.
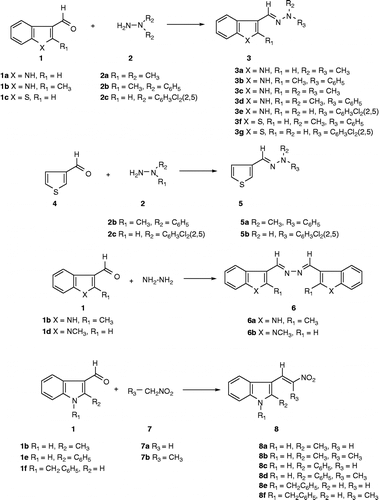
To study the structure-activity relationships of these compounds, we have synthesized a series of hydrazone and nitrovinyl analogs (figure 1) derived from a variety of substituted indole-3-carboxaldehydes, benzo[b]thiophen-3-carboxaldehyde and thiophene-3-carboxaldehyde, which were tested against M. tuberculosis, in order to uncover new leads in the development of novel antitubercular agents.
Materials and methods
All reagents utilized were of general purpose or analytical grade and were purchased from Aldrich Chemical Company (St. Louis, MO). 1H and 13C NMR spectra were recorded on a Varian Mercury-300 spectrometer, operating at 300 MHz and 75 MHz respectively, using an appropriate deuterated solvent (CDCl3 or DMSO-d6). Chemical shifts are given in parts per million (ppm) relative to the internal standard tetramethylsilane. GC-MS was recorded on an Agilent 6890 Series GC System interfaced to a 5973 Mass Selective Detector and utilizing a Fused Silica Capillary Column (30 m × 0.25 mm × 0.25 μm film thickness). Mass spectra were acquired by the University of Kentucky Mass Spectrometry Facility.
MALDI mass spectra were obtained on a Kratos Kompact SEQ time-of-flight mass spectrometer (Manchester, UK), using alpha-cyano-4-hydroxycinnamic acid as the matrix.
GC-Mass spectra were recorded on an Agilent 6890 GC incorporating an Agilent 7683 autosampler and an Agilent 5973 MSD (Agilent Technologies, Wilmington, DE) interfaced to a Hewlett-Packard KAYAK XM600 computer (Hewlett-Packard Co., Palo Alto, CA) with MSD Productivity ChemStation Software (Rev. C.00.01) (Agilent Technologies, Palo Alto, CA) for analysis and data processing. An HP-5MS GC column (30 m × 0.25 mm I.D. × 0.25 μm film thickness) (Agilent Co., Wilmington, DE) was utilized for analyses, with ultrapure helium as carrier gas at a flow rate of 1.0 mL/min and an average velocity of 38 cm/sec in constant flow mode. The temperature at the injection port, MS interface, electron impact source, and mass filter was set constant at 280°C, 280°C, 230°C, and 150°C, respectively. The oven temperature was initially set at 120°C and increased to 160°C at the rate of 10°C/min; after holding at 160°C for 1 min, the oven temperature was increased to 275°C at the rate of 60°C/min and held at this temperature for 10 min, to elute possible high boiling point interfering impurities. The MS analyzer was set in the scan mode.
High resolution electron impact ionization mass spectra were recorded at 70eV on a JEOL JMS-700T MStation (magnetic sector instrument) at a resolution of greater than 10,000. Samples were introduced via a heatable direct probe inlet. Perfluorokerosene (pfk) was used to produce reference masses.
Melting points were determined on a Fisher-Johns Scientific melting point apparatus and are uncorrected.
Chemistry
The general method for the preparation of hydrazones 3, 5 & 6 and nitrovinylindoles 8 is summarized in Scheme . Indole-3-carboxaldehyde (1a) was generally reacted under reflux with N,N-dimethylhydrazine (2a), N-methyl-N-phenylhydrazine (2b) and 2,5-dichlorophenylhydrazine (2c) to afford N′-(1H-indol-3-yl-methylene)-N,N-dimethylhydrazine (3a), (E)-N′-(1H-indol-3-ylmethylene)-N-methyl-N-phenylhydrazine (3b), and (E)-N-(2,5-dichlorophenyl)-N′-(1H-indol-3-ylmethylene)hydrazine (3e), respectively. Also, 2-methylindole-3-carboxaldehyde was reacted with 2a and 2b under identical conditions to yield N,N-dimethyl-N′-(2-methyl-1H-indol-3-ylmethylene)hydrazine (3c), (E)-N′-(2-methyl-1H-indol-3-ylmethylene)-N-phenylhydrazine (3d), respectively.
In a similar manner the sulfur isostere of indole-3-carboxaldehyde, benzo[b]thiophene-3-carboxaldehyde (1c), was reacted with 2b and 2c to afford (E)-N′-benzo[b]thiophen-3-yl-methylene-N-methyl-N-phenyl-hydrazine (3f) and (E)-N-benzo[b]-thiophen-3-ylmethylene-N′-(2,5-dichlorophenyl)hydrazine (3g), respectively. Additionally, thiophene analogs 5a and 5b were prepared by the reaction of thiophene-3-carboxaldehyde (4) with 2b and 2c to yield (E)-N-methyl-N-phenyl-N′-thiophen-3-ylmethylenehydrazine (5a), and (E)-N-(2,5-dichlorophenyl)-N′-thiophen-3-ylmethylene-hydrazine (5b), respectively. Unexpectedly, the reaction of 1b and 1d with hydrazine under similar conditions to those described above resulted in the formation of the dimeric compounds: N,N′-bis-(2-methyl-1H-indol-3-yl-methylene)hydrazine (6a) and N,N′-bis(1-methyl-1H-indol-3-ylmethylene)hydrazine (6b), respectively.
The nitrovinylindole analogs 8a-f were prepared by the reaction of indole-3-carboxaldehydes (1b, 1e & 1f) with nitromethane (7a) or nitroethane (7b) in the presence of ammonium acetate as the catalyst, to afford the desired nitrovinylindole, (E)-2-methyl-3-(2-nitrovinyl)-1H-indole (8a), (E)-2-methyl-3-(2-methyl-2-nitrovinyl)-1H-indole (8b), (E)-2-phenyl-3-(2-nitrovinyl)-1H-indole (8c), (E)-2-phenyl-3-(2-methyl-2-nitrovinyl)-1H-indole (8d), (E)-1-benzyl-3-(2-nitrovinyl)-1H-indole (8e), and (E)-1-benzyl-3-(2-methyl-2-nitrovinyl)-1H-indole (8f), in moderate yield (49–65%).
The structure and purity of the above indole derivatives were verified by 1H & 13C NMR spectroscopy, GC-MS and MALDI-TOF mass spectrometry. X-ray crystallographic analysis of four representative compounds has been previously reported [Citation15–17], in every case the C = N and C = C bonds had the E-geometry.
N′-(1H-Indol-3-yl-methylene)-N,N-dimethyl-hydrazine 3a
A mixture of indole-3-carboxaldehyde (1.45 g, 0.01 mol) and N,N-dimethylhydrazine (0.6 g, 0.01 mol) dissolved in ethanol (25 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from ethanol afforded N′-(1H-indol-3-yl-methylene)-N,N-dimethylhydrazine (72%) as pale yellow crystals: mp 101–103°C. 1H NMR (300 MHz, CDCl3): δ 2.93 (6H, s), 7.16–7.23 (2H, m), 7.29–7.36 (2H, m), 7.70 (1H, s), 8.19 (1H, sb), 8.29 (1H, d), 8.32 (1H, t) ppm; 13C NMR (75 MHz, CDCl3): δ 43.8, 111.1, 115.0, 120.6, 122.1, 123.0,124.3, 125.0, 132.1, 136.8 ppm. GC: tR = 9.88 min, purity>99% (single peak); MS (m/z) 187 (M+).
(E)-n′-(1h-indol-3-ylmethylene)-n-methyl-n-phenyl-hydrazine 3B
A mixture of indole-3-carboxaldehyde (1.45 g, 0.01 mol) and N-methyl-N-phenylhydrazine (1.22 g, 0.01 mol) dissolved in ethanol (25 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from ethanol afforded N′-(1H-indol-3-ylmethylene)-N-methyl-N-phenylhydrazine (64%) as pale yellow crystals: mp 104–106 °C. 1H NMR (300 MHz, DMSO-d6): δ 3.38 (3H, s), 6.8 (1H, t), 7.18 (2H, m), 7.27 (4H, m), 7.63 (1H, d), 8.0 (1H, s), 8.25 (1H, m), 11.25 (1H, s) ppm; 13C NMR (75 MHz, DMSO-d6): δ 32.27, 111.59, 113.46, 113.65, 118.59, 119.82, 121.21, 122.0, 123.91, 127.38, 128.85, 131.20, 136.79, 147.88 ppm. GC: tR = 15.77 min, purity>99% (single peak); MS (m/z) 249 (M+).
N,N-Dimethyl-N′-(2-methyl-1H-indol-3-ylmethylene)hydrazine 3c
A mixture of 2-methylindole-3-carboxaldehyde (1.59 g, 0.01 mol) and N,N-dimethylhydrazine (0.6 g, 0.01 mol) dissolved in ethanol (25 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from ethanol afforded N,N-dimethyl-N′-(2-methyl-1H-indol-3-ylmethylene)hydrazine (68%) as pale yellow crystals: mp 73–75 °C. 1H NMR (300 MHz, CDCl3): δ 2.54 (3H, s), 2.93 (6H, s), 7.16–7.22 (2H, m), 7.25–7.30 (1H, m), 7.75 (1H, s), 7.98 (1H, sb), 8.26–8.36 (1H, m) ppm; 13C NMR (75 MHz, CDCl3): δ 12.3, 44.0, 110.0, 110.2, 120.5, 121.3, 121.9, 126.3, 133.0, 134.8, 135.5 ppm. GC: tR = 10.58 min, purity>99% (single peak); MS (m/z) 201 (M+).
(E)-n-methyl-n′-(2-methyl-1h-indol-3-ylmethylene)-n-phenylhydrazine 3D
A mixture of 2-methylindole-3-carboxaldehyde (1.59 g, 0.01 mol) and N-methyl-N-phenylhydrazine (1.22 g, 0.01 mol) dissolved in ethanol (25 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and crystals that separated were collected by filtration. Recrystallisation from ethanol afforded (E)-N-methyl-N′-(2-methyl-1H-indol-3-ylmethylene)-N-phenylhydrazine (59%) as pale yellow crystals: mp 123–125°C. 1H NMR (300 MHz, DMSO-d6): δ 2.57 (3H, s), 3.42 (3H, s), 6.81 (1H, t), 7.10 (2H, h), 7.37 (5H, m), 7.97 (1H, s), 8.20 (1H, m), 11.22 (1H, s) ppm; 13C NMR (75 MHz, DMSO-d6): δ 11.84, 32.30, 108.86, 110.54, 113.53, 118.42, 119.40, 120.20, 121.05, 125.22, 128.83, 130.68, 135.40, 136.53, 148.02 ppm. GC: tR = 16.34 min, purity>99% (single peak); MS (m/z) 263 (M+).
(E)-N-(2,5-Dichlorophenyl)-N′-(1H-indol-3-ylmethylene)hydrazine 3e
A mixture of indole-3-carboxaldehyde (1.45 g, 0.01 mol) and 2,5-dichlorophenylhydrazine (1.77 g, 0.01 mol) dissolved in ethanol (25 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from ethanol afforded N-(2,5-dichlorophenyl)-N′-(1H-indol-3-ylmethylene)hydrazine (70%) as pale brown crystals: mp 163–165°C. 1H NMR (300 MHz, DMSO-d6): δ 6.75 (1H, dd), 7.20 (2H, m), 7.34 (1H, d), 7.45 (2H, q), 7.73 (1H, d), 8.16 (1H, t), 8.54 (1H, s), 9.68 (1H, s), 11.5 (1H, s) ppm; 13C NMR (75 MHz, CD3OD): δ 112.53, 114.01, 114.22, 115.75, 118.88, 121.49, 122.85, 123.77, 125.84, 128.91, 131.14, 134.75, 138.82, 140.51, 144.38 ppm. GC: tR = 12.09 min, purity>99% (single peak); MS (m/z) 303 (M+).
(E)-N′-Benzo[b]thiophen-3-ylmethylene-N-methyl-N-phenyl-hydrazine 3f
A mixture of benzo[b]thiophene-3-carboxaldehyde (0.487 g, 3 mmol) and N-methyl-N-phenylhydrazine (0.366 g, 3 mmol) dissolved in methanol (15 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from methanol afforded (E)-N′-benzo[b]thiophen-3-ylmethylene-N-methyl-N-phenylhydrazine (80%) as pale yellow crystals: mp 74–76°C. 1H NMR (300 MHz, CDCl3): δ 3.47 (3H, s), 6.97 (1H, tt), 7.35–7.52 (6H, m), 7.54 (1H, s), 7.87 (1H, s), 7.89 (1H, t), 8.79 (1H, dq) ppm; 13C NMR (75 MHz, CDCl3): δ 33.4, 115.6, 120.8, 122.7, 124.86, 124.94, 125.0, 125.9, 129.2, 129.3, 133.4, 136.4, 140.9, 148.1 ppm. GC: tR = 13.29 min, purity>99% (single peak); MS (m/z) 266 (M+).
(E)-N-Benzo[b]thiophen-3-ylmethylene-N′-(2,5-dichlorophenyl)hydrazine 3g
A mixture of benzo[b]thiophene-3-carboxaldehyde (0.487 g, 3 mmol) and 2,5-dichlorophenylhydrazine (0.531 g, 3 mmol) dissolved in methanol (15 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from methanol afforded (E)-N-benzo[b]thiophen-3-ylmethylene-N′-(2,5-dichlorophenyl)hydrazine (78%) as brown crystals: mp 148–150°C. 1H NMR (300 MHz, CDCl3): δ 6.78 (1H, dd), 7.42 (1H, d), 7.44 (1H, t), 7.55 (1H, t), 7.59 (1H, d), 7.61 (1H, s), 7.88 (1H, d), 8.00 (1H, s), 8.69 (1H, d) ppm; 13C NMR (75 MHz, CDCl3): δ 114.0, 114.01, 115.2, 119.9, 122.8, 124.9, 125.3, 125.4, 128.8, 130.1, 131.4, 134.1, 136.0, 137.1, 140.8, 141.4 ppm. GC: tR = 16.39 min, purity>99% (single peak); MS (m/z) 320 (M+).
(E)-N-Methyl-N-phenyl-N′-thiophen-3-ylmethylenehydrazine 5a
A mixture of thiophene-3-carboxaldehyde (0.336 g, 3 mmol) and N-methyl-N-phenylhydrazine (0.366 g, 3 mmol) dissolved in methanol (15 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from methanol afforded (E)-N-methyl-N-phenyl-N′-thiophen-3-ylmethylenehydrazine (82%) as pale yellow crystals: mp 80–82°C. 1H NMR (300 MHz, CDCl3): δ 3.39 (3H, s), 6.91–6.96 (1H, m), 7.29–7.38 (6H, m), 7.58 (1H, dd), 7.60 (1H, s) ppm; 13C NMR (75 MHz, CDCl3): δ 33.4, 115.3, 120.6, 122.3, 125.3, 126.1, 128.2, 129.1, 140.0, 147.9 ppm. GC: tR = 10.38 min, purity>99% (single peak); MS (m/z) 216 (M+).
(E)-N-(2,5-Dichlorophenyl)-N′-thiophen-3-ylmethylenehydrazine 5b
A mixture of thiophene-3-carboxaldehyde (0.336 g, 3 mmol) and 2,5-dichlorophenylhydrazine (0.531 g, 3 mmol) dissolved in methanol (15 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from methanol afforded (E)-N-(2,5-dichlorophenyl)-N′-thiophen-3-ylmethylenehydrazine (79%) as brown crystals: mp 94–96°C. 1H NMR (300 MHz, CDCl3): δ 6.75 (1H, dd), 7.17 (1H, d), 7.35 (1H, q), 7.43 (1H, dd), 7.55 (1H, t), 7.57 (1H, d), 7.89 (2H, s) ppm; 13C NMR (75 MHz, CDCl3): δ 114.1, 115.1, 119.8, 124.9, 125.2, 126.7, 130.0, 134.0, 136.3, 137.7, 141.4 ppm. GC: tR = 11.43 min, purity>99% (single peak); MS (m/z) 270 (M+).
N,N′-bis-(2-Methyl-1H-indol-3-yl-methylene)hydrazine 6a
A mixture of 2-methylindole-3-carboxaldehyde (1.59 g, 0.01 mol) and hydrazine (0.64 g, 0.02 mol) dissolved in ethanol (25 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from methanol afforded N,N′-bis-(2-methyl-1H-indol-3-yl-methylene)hydrazine (65%) as yellow crystals: mp>300°C. 1H NMR (300 MHz, DMSO-d6): δ 3.25 (6H, s), 7.00–7.03 (4H, m), 7.22–7.25 (2H, m), 8.21 (2H, d), 8.83 (2H, s), 11.44 (2H, s) ppm; 13C NMR (75 MHz, DMSO-d6): δ 11.7, 107.8, 110.7, 120.2, 121.4, 121.6, 125.8, 135.6, 141.3, 153.7 ppm. MALDI-MS (m/z) 315 (MH+).
N,N′-bis-(1-Methyl-1H-indol-3-yl-methylene)hydrazine 6b
A mixture of 1-methylindole-3-carboxaldehyde (1.59g, 0.01 mol) and hydrazine (0.64 g, 0.02 mol) dissolved in ethanol (25 mL) was heated under reflux for 2 h. The reaction mixture was cooled to room temperature and the crystals that separated were collected by filtration. Recrystallisation from methanol afforded N,N′-bis-(2-methyl-1H-indol-3-yl-methylene)hydrazine (62%) as yellow crystals: mp 224–226°C. 1H NMR (300 MHz, DMSO-d6): δ 3.84 (6H, s), 7.18–7.30 (4H, m), 7.51 (2H, d), 7.88 (2H, s), 8.34 (2H, dd), 8.86 (2H, s) ppm; 13C NMR (75 MHz, DMSO-d6): δ 32.9, 110.2, 111.0, 120.7, 122.1, 122.6, 125.0, 135.2, 137.6, 154.5 ppm. MALDI-MS (m/z) 315 (MH+).
General method for the preparation of 3-(2-nitrovinyl)indoles 8a-f
Solid ammonium acetate (0.3 g, 3.8 mmol) was added to a suspension of the appropriately substituted indole-3-carboxaldehyde (6.8 mmol) in nitromethane or nitroethane (3 mL). The mixture was vigorously stirred and heated at 120–130°C for 2 h. The mixture was then cooled in an ice bath and the resulting solid which appeared was filtered and recrystallised from methanol.
(E)-2-Methyl-3-(2-nitrovinyl)-1H-indole 8a
Yellow crystals (62%): 190–192°C. 1H NMR (300 MHz, DMSO-d6): δ 2.60 (3H, s), 7.15–7.22 (2H, p), 7.38–7.42 (1H, m), 7.80–7.84 (1H, m), 7.88 (1H, d), 8.30 (1H, d), 12.20 (1H, b) ppm; 13C NMR (75 MHz, DMSO-d6): δ 11.93, 105.08, 111.79, 120.01, 121.80, 122.72, 125.10, 129.50, 133.19, 136.24, 147.55 ppm. GC: tR = 12.51 min, purity>99% (single peak); MS (m/z) 202 (M+).
(E)-2-Methyl-3-(2-methyl-2-nitrovinyl)-1H-indole 8b
Brown crystals (56%): mp 154–156°C. 1H NMR (300 MHz, DMSO-d6): δ 2.25 (3H, s), 2.42 (3H, s), 7.08–7.13 (2H, pd), 7.37 (1H, dd), 7.47 (1H, dd), 8.28 (1H, s), 11.92 (1H, s) ppm; 13C NMR (75 MHz, DMSO-d6): δ 12.58, 15.82, 105.29, 111.45, 119.39, 120.30, 121.65, 125.57, 129.12, 135.86, 141.46, 141.63 ppm. GC: tR = 11.63 min, purity>99% (single peak); MS (m/z) 216 (M+).
(E)-2-Phenyl-3-(2-nitrovinyl)-1H-indole 8c
Yellow crystals (58%): mp 220–222°C. 1H NMR (300 MHz, DMSO-d6): δ 7.24–7.35 (2H, m), 7.53 (1H, d), 7.57–7.68 (5H, m), 8.0 (1H, d), 8.06 (1H, dd), 8. 15 (1H, dd), 12.60 (1H, b) ppm; 13C NMR (75 MHz, DMSO-d6): δ 104.93, 112.47, 120.94, 122.23, 123.78, 125.07, 129.03, 129.61, 129.74, 129.88, 131.52, 133.90, 136.89, 147.70 ppm. GC: tR = 17.41 min, purity>99% (single peak); MS (m/z) 264 (M+).
(E)-2-Phenyl-3-(2-methyl-2-nitrovinyl)-1H-indole 8d
Orange crystals (49%): mp 228–230°C. 1H NMR (300 MHz, DMSO-d6): δ 2.20 (3H, s), 7.14–7.27 (2H, m), 7.46–7.64 (7H, m), 8.19 (1H, s), 12.28 (1H, b) ppm; 13C NMR (75 MHz, DMSO-d6): δ 15.65, 105.03, 112.12, 119.98, 120.67, 122.75, 125.89, 128.67, 128.86, 129.17, 131.00, 136.47, 141.17, 144.03 ppm. GC: tR = 15.71 min, purity>99% (single peak); MS (m/z) 278 (M+).
(E)-1-Benzyl-3-(2-nitrovinyl)-1H-indole 8e
Yellow crystals (65%): mp 128–130°C. 1H NMR (300 MHz, DMSO-d6): δ 5.52 (2H, s), 7.25–7.34 (7H, m), 7.63 (1H, dd), 7.99–8.06 (2H, m), 8.40 (2H, dd) ppm; 13C NMR (75 MHz, DMSO-d6): δ 49.73, 107.78, 111.58, 120.72, 122.13, 123.40, 125.16, 127.13, 127.65, 128.59, 131.41, 133.87, 136.56, 137.33, 138.55 ppm. GC: tR = 17.15 min, purity>99% (single peak); MS (m/z) 278 (M+).
(E)-1-Benzyl-3-(2-methyl-2-nitrovinyl)-1H-indole 8f
Yellow crystals (63%): mp 114–116°C. 1H NMR (300 MHz, DMSO-d6): δ 2.48 (3H, s), 5.56 (2H, s), 7.19–7.31 (7H, m), 7.55 (1H, dd), 7.86 (1H, dd), 8.31 (1H,s), 8.44 (1H, dd) ppm; 13C NMR (75 MHz, DMSO-d6): δ 14.83, 49.84, 107.70, 111.22, 118.51, 121.30, 123.05, 125.86, 127.09, 127.54, 128.12, 128.53, 133.04, 135.78, 136.93, 141.18 ppm. GC: tR = 16.69 min, purity>99% (single peak); MS (m/z) 292 (M+).
Microbiology
All compounds were evaluated for antimycobacterial activity against Mycobacterium tuberculosis H37Rv. Antitubercular activities of the compounds were performed by the Center of Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) at Southern Research Institute. Compounds were tested for in vitro antituberculosis activity against Mycobacterium tuberculosis H37Rv (ATCC 27294) at 6.25 μg/mL, in BACTEC 12B medium using a broth microdilution assay, the Microplate Alamar Blue Assay (MABA). Compounds exhibiting fluorescence were tested in the BACTEC 460 Radiometric System [Citation18,Citation19]. Compounds effecting < 90% inhibition in the primary screen (i.e., MIC>6.25 μg/mL) were generally not evaluated further. Compounds exhibiting growth inhibition of>90% in the primary screen at 6.25 μg/mL were retested at lower concentration against M. tuberculosis H37Rv to determine the actual Minimum Inhibitory Concentration (MIC) in the broth microdilution MABA assay. The MIC is defined as the lowest concentration effecting a reduction in fluorescence of 90% relative to controls. Also the cytotoxicity (IC50) of compounds against cultured VERO cells, as well as their selectivity index (SI), defined as IC50/MIC, were determined. Rifampicin was utilized as the standard compound in these assays.
Results and discussion
As shown in , the compounds 3b, 3d, 3e, 3g and 8b exhibited growth inhibition>90% in the primary screen at 6.25 μg/mL, and were therefore retested at lower concentration against M. tuberculosis H37Rv to determine the actual Minimum Inhibitory Concentration (MIC) in the MABA assay.
Table I. Primary antitubercular activity screening results of 3a-g, 5a-b, 6a-b & 8a-f. and IC50 values for 3b, 3d, 3e & 8b.
Also, the cytotoxicity (IC50) of compounds 3b, 3d, 3e & 8b against VERO cells as well as their selectivity index (SI), were determined. Unfortunately the solubility of compound 3g in the tissue culture medium was too low for an IC50 to be determined.
Based on the data from the antitubercular MABA assays for compounds 3a-g, 5a, 5b, 6a, 6b, and 8a-f, using M. tuberculosis H37RV the following observations can be made. In the indole series, hydrazones 3a and 3c derived from N,N-dimethylhydrazine did not exhibit any significant growth inhibition (i.e. 9% and 0% inhibition, respectively). However, the compounds derived from N-methyl-N-phenylhydrazine in the indole series (i.e. 3b and 3d) were active, exhibiting 91% & 95% growth inhibition of M. tuberculosis H37Rv, respectively. Thus, it appears that the presence of an N,N-dimethyl moiety in the molecule affords poor inhibitory activity; however, when one of the N-methyl groups is replaced with a phenyl or substituted phenyl group, inhibitory activity is greatly improved. It is also apparent that the presence or absence of an indolic 2-methyl substituent has no effect on inhibitory potency. Interestingly, compound 3e, which is a mono N-2,5-dichlorophenyl substituted hydrazone, was the most potent compound (IC50 = 5.4 μg/mL) in the series, suggesting that N-aryl mono substitution may be optimal for antitubercular activity in this series of indole hydrazones.
The benzo[b]thiophene analog 3g, which is isosteric with 3e (i.e. the only difference is the replacement of the indole NH moiety with an S atom), exhibited potent inhibitory activity against M. tuberculosis H37Rv, further establishing that a mono substituted N-2,5-dichlorophenyl substituent is a requirement for potent inhibitory activity in both the indole and benzo[b]thiophene series of analogs. However, when the indole moiety in the N-methyl-N-phenyl analog 3b is replaced with a benzo[b]thiophene moiety (3f), there is considerable loss in inhibitory activity (i.e. 91% for 3b, decreasing to 34% for 3f). We also observed that replacing the benzo[b]thiophene moiety in 3g with a thiophene moiety (5b) still maintained moderate but reduced inhibitory activity (i.e. 96% versus 70% inhibition, respectively). However, replacement of the indole moiety in 3b with a thiophene moiety to afford 5a, resulted in a significant loss of inhibitory activity. Interestingly, none of the bis analogs 6a and 6b exhibited any significant inhibitory activity in the MABA assay.
These results suggest that hydrazones derived from aromatic aldehydes possessing a 6 + 4π electron system, and having one N-phenyl or substituted N-phenyl group on the terminal nitrogen affords compounds with maximum inhibitory activity as antitubercular agents. The most active compounds contained an N-2,5-dichlorophenyl moiety.
The nitrovinyl analogs 8a-8f also were evaluated in the MABA assay for antitubercular activity. The results indicate that the 2-methylindole analog 8b was the most potent compound exhibiting 91% inhibition in the primary screen. Replacing the 2-methyl group in compound 8b with a 2-phenyl group resulted in compound 8d, which had reduced inhibitory activity, suggesting that introduction of a bulky 2-substituent into the indole nucleus leads to a significant reduction in inhibitory activity. Additionally, introduction of an N-benzyl moiety into the 8b molecule to afford 8f resulted in a significant loss of inhibitory activity, suggesting that steric bulk at the indolic N-atom also results in loss of inhibitory potency.
Concurrent with the determination of MIC values, compounds which were active (i.e. MIC′s> 90%) were also tested for cytotoxicity (IC50) in VERO cells at concentrations less than or equal to 62.5 μg/mL, or 10 times the MIC for M. tuberculosis H37Rv. After 72 hours exposure, viability was assessed on the basis of cellular conversion of MTT into a formazan product using the Promega CellTiter 96 non-radioactive cell proliferation assay. Compounds 3b, 3d, 3e, & 8b afforded IC50 values of>10, 5.96, 5.40, and 1.6 μg/mL, respectively, in the cytotoxicity assay. Compound 3g was not evaluated due to solubility problems.
These preliminary studies have identified three lead molecules 3d, 3e, & 8b from both the hydrazone and nitrovinyl series of indole derivatives investigated. The nitrovinyl analog 8b was the most potent compound examined, and was approximately one-tenth as active as rifampin in the VERO cell assay for cytotoxicity. Compounds 3d, 3e, & 8b represent potential lead molecules for the further development of novel antitubercular agents.
Acknowledgements
The authors thank the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF), National Institute of Allergy and Infectious Diseases Southern Research Institute, GWL Hansen′s Disease Center, Colorado State University, Birmingham, AL, USA, for the in vitro evaluation of antimycobacterial activity using M. tuberculosis H37RV.
References
- MC Raviglione, DE Snider, and A Kochi. (1995). J Am Med Assoc 273:220–226.
- A Kochi. (1991). Tubercle 72:1–6.
- N Lounis, B Ji, C Truffot-Pernot, and J Grosset. (1997). Antimicrob Agents Chemother 41:607–610.
- D Libermann, M Moyeux, A Rouaix, J Maillard, L Hengl, and J Himbert. (1953). Bull Soc Chim Fr 957–962.
- LE Weller, HM Sell, and RY Gottshall. (1954). J Am Chem Soc 76:1959.
- SM Sodhi, M Dinodia, and A Kumar. (2000). Bioorg Med Chem 14:4657–4663.
- R Gatti, V Cavrini, P Roveri, F Bianucci, and P Legnani. (1981). Farmaco. Edizione Scientifica 36:102–108.
- JC Agarwal, YK Gupta, and K Shanker. (1984). Pharmazie 39:775.
- L Garuti, G Giovanninetti, A Ferranti, A Chiarini, G Bertocchi, P Sabatino, and P Brigidi. (1987). Pharmazie 42:378–381.
- FD Kopp. (1989). Eur J Med Chem 24:313–315.
- F Micheli, R Di Fabio, D Baraldi, N Conti, A Cugola, P Gastaldi, S Giacobbe, C Marchioro, M Mugnaini, L Rossi, A Pecunioso, and G Pentassuglia. (1999). Archiv der Pharmazie 332:271–278.
- AL Mindzhoyan, GL Papayan, LD Zhuruli, SG Karagezyan, LS Galstyan, and VG Sarafyan. (1969). Arm Khim Zh 22:707–713.
- L Canoira, RJ Gonzalo, JB Subirats, JA Escario, I Jimenez, and AR Martinez-Fernandez. (1989). Eur J Med Chem 24:39–42.
- GL Sharma, S Mukhopadhya, R Kaur, and SK Banjerjee. (1987). Indian J Med Res 86:783–786.
- VN Sonar, S Parkin, and PA Crooks. (2004). Acta Cryst C60:o550–o551.
- VN Sonar, S Parkin, and PA Crooks. (2005). Acta Cryst E61:o1907–o1909.
- VN Sonar, S Parkin, and PA Crooks. (2006). Acta Cryst C61:o527–o530.
- LA Collins, and SG Franzblau. (1997). Antimicrob Agents Chemother 41:1004–1009.
- BP Kelly, SK Furney, MT Jessen, and IM Orme. (1996). Agents Chemother 40:2809–2812.
- SW Yee, B Shah, and C Simons. (2005). J Enz Inhib 20:109–113.