Abstract
The whole plant of genus Carpesium is used in traditional medicine as an anti-pyretic, analgesic and vermifugic, including a topical application for sores and inflammation. Previous experiments on Carpesium rosulatum suggested that the antiplasmodial effect was due to the existence of ineupatorolide A. In present paper, screening of Carpesium species from South Korea showed that this plant refers to which species had promising antiplasmodial activity. Subsequently, this species was selected for bioassay-guided fractionation in order to identify the active principles. Fractionation of the ethyl acetate extract of the whole plants by chromatographic techniques yielded four characterised sesquiterpenoid lactones which exhibited antiplasmodial activity against Plasmodium falciparum. This being the first time that this has been reported from Carpesium cernum. The antiplasmodial activity of the isolated compounds was determined against the Plasmodium falciparum.
Introduction
Malaria is still the most important parasitic disease in the world, causing 2–3 million deaths every year [Citation1]. The increased threat represented by malaria based on the rapid spread of parasite resistance to the cheap and previously very effective antimalarial drug chloroquine, means there is an urgent need to find new antiplasmodial agents. Among the four species of Plasmodium that infect humans, Plasmodium falciparum is responsible for the most severe cases of malaria. There is, therefore, an urgent need to discover and develop new, effective and safe drugs for the treatment of this disease [Citation2]. As most of the antimalarial agents in widespread use have been derived from medicinal plants and/or from structures modelled on plant derived lead compounds, the possibility that new drugs could be based on other compounds from plants is being investigated by several research teams. There is an urgent need for new inexpensive antimalarial compounds and in vitro screens of plants commonly used in traditional healthcare can be considered key components of a critical path for antimalarial drug discovery. Existing botanical, pharmacological and ethno-pharmacological data can, if accurate, greatly help in narrowing the search [Citation3].
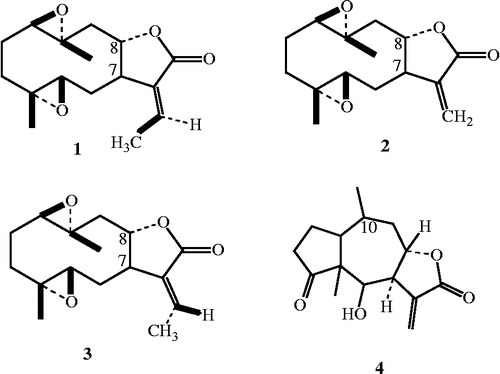
The genus Carpesium is widespread in South Korea, has been used in Korean traditional medicine for its anti-pyretic, analgesic, vermifugic, and anti-inflammatory properties [Citation4]. Maruyama et al. reported the isolation of several sesquiterpene lactones from the genus Carpesium; granilin [Citation5], carpesiolin, carabrone [Citation6], carabrol, ivaxillin [Citation7], and ineupatolides A and B from C. abrotanoides [Citation8], and divaricins A, B, and C from C. divaricatum [Citation9]. Kim et al. reported the isolation of several cytotoxic sesquiterpene lactones; cardivins A, B, C, and D, from C. divaricatum [Citation10]. Kim et al. reported the isolation of two new guaianolides from C. macrocephalum [Citation11]. Moon. reported the isolation, structure elucidation and in-vitro antimalarial activity of a sesquiterpene lactone from an chloroform - soluble fraction of the aerial part from C. rosulatum [Citation12]. In this paper, screening of Carpesium species from South Korea showed that this plant had promising antiplasmodial activity. These were pooled by EtOAc extracts, of which C. cernum was the most active (). As there are no previous reports on the chemical components of C. cernum, the plant was studied to isolate and characterise the major active compounds responsible for its antiplasmodial properties. The bioassay-guided fractionation of the extract led to the isolation of 1–4 () identified as sesquiterpene lactones of the germacranolide and pseudoguaianolide type, previously isolated from other members of Carpesium species [Citation6,Citation7]. Structural elucidation was facilitated by spectroscopic data (NMR spectroscopy and mass spectrometry) and by direct comparison with published spectral data. This is the first account of the antiplasmodial properties of compounds 1–4.
Table I. In vitro antiplasmodial activity of ethyl acetate extract of Carpesium genus in southern part of Korea against Plasmodium falciparum (D10) strain.
Materials and methods
General experimental procedures
Optical rotations: Jasco DIP-1000 polarimeter; ultraviolet (UV): UV-2200 UV–VIS recording spectrophotometer (Shimadzu, Japan); infrared (IR): Jasco Report-100 spectrophotometer; nuclear magnetic resonance (NMR): Bruker AMX 400 spectrometer (Bruker, Germany), the chemical shifts being represented as parts per million (ppm) with tetramethylsilane as an internal standard; positive-ion fast atom bombardment mass spectroscopy (FAB-MS): JMS-AX 110/110A (Jeol, Japan); preparative high-performance liquid chromatography (HPLC) was carried out on a Shimadzu system: LC10AD pump, SPD-10AV UV detector; gas chromatography-(GC)-Mass (HP 5890 series II plus GC, HP 5972 series mass selective detector, column: HP-1MS); column chromatography (cc): silica gel 60 (70 ∼ 230 and 230 ∼ 400 mesh, Merck), Sephadex LH-20 (Pharmacia, Sweden), and YMC-GEL ODS-A (12 nm, S-75 mm, YMC); thin layer chromatography (TLC): pre-coated silica gel 60 F254. Artemisinin was purchased from Sigma-Aldrich (St. Louis, MO).
Plant materials
Carpesium divaricatum was collected in August 1998, at Samyeong Mt., Kang-won-do, South Korea. Carpesium abrotanoides, Carpesium macrocephalum, Carpesium glossophyllum and Carpesium triste were collected in August 1999, at O-Dae Mt., Kang-won-do, South Korea. Carpesium cernum was collected in August 2003, at Ue-Wang Mt, Kyung-sag-buk-do, South Korea. Carpesium rosulatum were collected in August 2004, at Ul-Reung island Kyung-sag-buk-do, South Korea. Voucher specimens are deposited at the herbarium of Sung Kyun Kwan University (Suwon, South Korea). The botanical identification was made by Dr. Kim Tae-Jin (KRIBB in Daejeon, Korea). All voucher numbers are indicated in .
Extraction and bioassay-guided fractionation
For antimalarial bioassay from EtOAc extract, the dried and powdered plant material (10 g) was impregnated with 100 mL of a mixture of EtOAc–methanol–NH4OH (2:1:1), macerated during 4 h on a sonication bath at 35°C and then extracted by EtOAc (150 mL), filtered and evaporated to dryness under reduced pressure below 40°C to yield a crude EtOAc extract. In vacuo evaporation of solvents from the filtered EtOAc extracts gave residues, which were subjected to an immediate lyophilization into dry powders with the yields given in . For the bioassay, the fractions were dissolved in dimethyl sulfoxide and further diluted with incubation buffer. These were again pooled by EtOAc extracts, of which C. cernum was the most active (). Therefore, active compound was isolated from C. cernum. In the case of the most active species, another quantity of material (200 g) was also extracted by methanol (3 mL × 25 mL), filtered and evaporated to dryness in the same way. The air-dried whole plants from C. cernum (200 g) were extracted with EtOAc–methanol–NH4OH (2:1:1) by refluxing for 4 h (3 times × 0.5 L) on a sonication bath at 35°C. The extract was filtered through a Buchner funnel using Whatman no. 1 filter paper. Vacuum Liquid Chromatography (Merck 9385, 150 g, 6 × 30 cm) of the EtOAc extract (13 g), using hexane - CH2Cl2 (1:0–0:1) and CH2Cl2 - MeOH (1:0–0:1) step gradients, produced 16 fractions. These were pooled by TLC profile into 6 fractions (CC 1∼ CC 6) and subjected to antimalarial bioassay, in which fraction CC 3 (292.3 mg) eluted with hexane - CH2Cl2 (1:1, 1:2, 1:4, 1:7 and only CH2Cl2), was found to be the most active (). The CC-3-2 subfraction (135 mg), which exhibited antiplasmodial activity, was rechromatographed with silica column using n-hexane - CH2Cl2 - MeOH (20:30:1) and sephadex LH-20 Column using acetone - MeOH (1:5). The four compounds were purified by preparative HPLC (Econosil C-18, 10 × 250 mm; 1.0 mL/min) with MeCN = MeOH (3:1–1:2) to afford compound 1 (7 mg), 2 (6 mg), 3 (11 mg) and 4 (8 mg). Throughout the isolation procedure, antiplasmodial activity was monitored using a chloroquine-sensitive strain of P. falciparum (D10). All experiments were performed in duplicate on a single occasion and repeated twice. In the assays of antimalarial activity, artemisinin was used as the positive control. Tests for antimalarial activity were carried out as described earlier [Citation13]. The results were expressed by the inhibitory concentrations 50% (IC50) and 90% (IC90), representing the concentration of drug that induced, respectively, a 50% and a 90% parasitemia decrease compared to control culture. The IC50 value of chloroquine diphosphate (Sigma), used as an antiplasmodial reference was determined as 0.02 μM. The parasites were continuously cultured according to the methods described by Trager and Jensen [Citation14] and the parasite lactate dehydrogenase activity was used as a measure of parasite viability [Citation15]. The results are expressed as the average percentage parasite viability at three different concentrations. The median inhibitory concentration (IC50 and IC90) for each such sample was then estimated from the dose–response curve, using the SigmaPlot ® software package (Systat, San Jose, CA).
Tabel II. In vitro antiplasmodial activity (percentage of parasite viability) of a fractionated ethyl actate extract of Carpesium cernum against Plasmodium falciparum (D10) strain.
MTT assay
Cell lines purchased from ATCC (Manassas, VA, USA) were maintained in Dulbecco's modified Eagle's media (DMEM) supplemented with 10% horse serum and 5% fetal bovine serum and incubated in a CO2 incubator (5%) at 37°C. Cells were serum-deprived by three washes of PBS and resuspended in DMEM. The suspended cells were plated on 96-well plates (1 × 104 cells/well) and treated with the indicated reagent(s). After treatment for 21 h, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was added to the medium (0.5 mg/mL), and the mixture was incubated at 37°C for another 3 h. After discarding the medium, DMSO (100 mL) was then applied to the well to dissolve the formazan crystals, and the absorbances at 570 and 630 nm in each well were measured on a micro-ELISA reader.
Identification of compounds 1–4
The bioassay-guided fractionation process yielded compounds 1–4. Compounds were identified using spectral data such as 1H, 13C, HMBC and COSY NMR spectra, as well as EI-MS spectra, and by direct comparison with published spectral data and structures. NMR spectra were recorded in CDCl3 and/or acetone-d6 using a Bruker AMX 400 MHz spectrophotometer. Mass spectra were recorded on a Jeol - MS instrument. Compound 1 yielded a crystalline substance, mp 177-178°C, C15H22O4. The 1H NMR and 13C NMR spectral data of this substance were very similar to those of ivaxillin (1) [Citation7], and their IR spectra were superimposable (). Therefore, this substance was identified as 1. 2 also yielded a crystalline substance, mp 166-167°C, C15H2O4. 2 had an a-methylene-γ-lactone instead of the secondary methyl group and γ -lactone found in 1. The rest of its 1H NMR spectrum was very similar to that of 1. Hydrogenation of 2 over platinum catalyst afforded two products, one of which was identified as 1. Therefore, 2 was established as 11(13)-dehydroivaxillin [Citation7]. The other product 3, mp 218-220°C, C15H22O4, was a C-11 epimer of ivaxillin [Citation7]. 4 also yielded a crystalline substance, mp 122-123°C, C15H20O4. The 1H NMR and 13C NMR spectral data of this substance were very similar to those of carpesiolin (4) [Citation8], and their IR spectra were superimposable. Therefore, this substance was identified as 4.
Results and discussion
The IC50 and IC90 values for each dry plant extract are shown in . The different total extracts displayed a wide range of antiplasmodial activities. We decided to consider an
IC50 between 30 and 15 μg/mL as a weak activity, between 15 and 5 μg/mL as a moderate activity and an IC50 under 5 μg/mL as an important activity for an extract. In view of these results, it appeared that among the 7 tested species, C. rosulatum, C. divaricatum and C. cern could be suitable for a bioguided separation of their active compounds, even if they were similar active with the previously studied C. rosulatum (IC50 = 5.2 μg/mL, whole plant, EtOAc extract). The highest activity was obtained with whole plant extract from C. cernum: the IC50 value was about 3.1 μg/mL with an IC90 close to 5.2 μg/mL. Whole plants of C. glossophyllum, C. abrotanoides, C. macrocephalum and C. triste showed a moderate and/or weak activity against Plasmodium falciparum. The crude EtOAc extract of the whole plants of C. cernum had an in vitro IC50 of 3.1 μg/mL against the chloroquine-sensitive D10 strain. Bioassay-guided fractionation led to the identification of four structurally related compounds responsible for the observed activity of this extract. It was evident by TLC and HPLC analysis that less active analogues of these compounds were present in the crude extract and that the overall antiplasmodial activity of the extract and several of the fractions generated were due to the synergistic effect of a number of compounds. Purification was, however, focused on those fractions showing significant enrichment of antiplasmodial activity upon fractionation so as to isolate the compounds primarily responsible for the observed biological activity. Also, isolation of additional compounds from active fractions proved unsuccessful due to low yields and marked instability. 1, 2 and 3 were identified as germacranolides by their lactone and cyclodecane rings, as well as the exocyclic double bond conjugated with the lactone carbonyl. They were characterised as germacrane lactones with a linear structure due to the α, β -unsaturated lactone being fused to the C-7, 8 positions of the carbocyclic skeleton. 4 was identified as the pseudoguaianolide derivative, known as carpesiolin. Although 1–4 are all known compounds, this is the first report of their isolation from Carpesium cernum. Once it was established that compounds 1–3 all possessed an α-methylene-γ-lactone functional group, which is typically responsible for the biological activity of sesquiterpene lactones [Citation16], an attempt was made to determine what effect the reduction of the C-11, 13 exocyclic double bond would have on the antiplasmodial activity and cytotoxicity of these compounds. Testing of 1–4 for in vitro antiplasmodial activity against the Plasmodium falciparum D10 trains using the pLDH assay revealed that the germacranolides 1, 2 and 3 were significantly more active than the pseudoguaianolide (4) (). This is most likely due to the flexibility and conformational features of the 10 - membered ring as opposed to the bi-cyclohexane ring system but the effect of other structural features cannot be ruled out. There are no previous reports of 1, 2 and 3 being investigated for any biological activity. 4 has been found to show cytotoxic activity [Citation17]. This is the first report of any of the compounds having antiplasmodial activity. In order to determine the specificity of the antiplasmodial activity of the sesquiterpene lactones, it was considered necessary to obtain information about their general cytotoxicity. Therefore, the compounds were tested for cytotoxicity against an adenocarcinoma (SK-OV-3) cell line, using the tetrazolium (MTT) assay. The corresponding IC50 and SI (selectivity index) values of chloroquine and the four compounds are listed in . In considering a recent publication [Citation18] outlining criteria for antiparasitic drug discovery, a compound can be considered a hit if it is active in vitro against whole protozoa with an IC50 of ≤ 1μg/mL and selective if it is at least 10-fold more active against the parasite than against a mammalian cell line. Based on these criteria, only 2 can be considered a hit. 1, 3 and 4 were not active enough. Overall, the data suggests that the observed antiplasmodial activity might be due to general toxicity. Since 1, 2 and 3 all possess an α-methylene-γ-lactone moiety; one would expect that they would all show equipotent antiplasmodial activity and toxicity to SK-OV-3 cells, which is clearly not the case. The fact that there are significant differences in the IC50 values of each compound in the two assays as well as between the various compounds suggests that something else is going on her besides the cytotoxic effect of the α-methylene-γ-lactone group. The identification of antiplasmodial sesquiterpene lactones from C. cernum suggests that they may play a role in the medicinal properties of the plant. These compounds could also be used as scaffolds to generate leads with enhanced antiplasmodial activity, reduced cytoxicity and improved bioavailabilty. Further SAR studies would also help draw a conclusion as to whether the antiplasmodial activity observed for sesquiterpene lactones such as 1, 2, 3 and 4 is indeed biological activity or just the result of general cytotoxicity. and, we will attempt to scale up our isolation process to support in vivo testing and mechanistic studies.
Tabel III. In vitro antiplasmodial activity, cytotoxicity and selectivity index (SI) values for chloroquine and compounds 1–4.
References
- C Clarkson, VJ Maharaj, and NR Crouch. (2004). J Ethnopharmacol. 92:177.
- B Greenwood, and T Mutabingwa. (2002). Nature. 415:670.
- A Petelot. Les plantes médicinales du Cambodge, du Laos et du Vietnam. In: I Tome, editor. Saigon: Centre de Recherches Scientifiques et Techniques. I.D.E.O; (1952). p 167–168.
- CS Yook. Medicinal plants of Korea. Seoul: Jinmyeong Publishing Co; (1981). p 392.
- M Maruyama, and F Shibata. (1975). Phytochemistry. 14:2247.
- M Maruyama, and S Omura. (1977). Phytochemistry. 16:782.
- M Maruyama, A Karube, and K Sato. (1983). Phytochemistry. 22:2773.
- M Maruyama, K Watanabe, T Kawakami, M Maeda, M Kato, S Nozoe, and T Ohta. (1995). Planta Med. 61:388.
- M Maruyama. (1990). Sesquiterpene lactones from Carpesium divaricatum. Phytochemistry. 29:547.
- DK Kim, NI Baek, SU Choi, CO Lee, KR Lee, and OP Zee. (1997). J Nat Prod. 60:1199.
- MR Kim, CS Kim, KH Hwang, IY Park, SS Hong, JK Son, and DC Moon. (2002). J Nat Prod. 65:583.
- HI Moon. (2007). Parasitol Res. 100:1147.
- M Frederich, MC De Pauw, C Prosperi, M Tits, V Brandt, J Penelle, MP Hayette, L Angenot, and J De Mol. (2001). Nat Pro. 64:12.
- W Trager, and JB Jensen. (1976). Science. 193:673.
- MT Makler, JM Ries, JA Williams, JE Bancroft, RC Piper, BL Gibbins, and DJ Hinrichs. (1993). Am J Trop Med Hyg. 48:739.
- E Rodriguez, GHN Towers, and JC Mitchel. (1976). Phytochemistry. 15:1573.
- J Lee, B Min, S Lee, M Na, B Kwon, C Lee, Y Kim, and K Bae. (2002). Planta Med. 68:745.
- R Pink, A Hudson, M Mouries, and M Bendig. (2005). Nature Rev-Drug Disc 4:727.