Abstract
The active fraction extracted from dragon's blood displayed an inhibitory effect on alpha-glucosidase activity with an IC50 of 0.152 μg/mL, which is nearly half of the crude material. Its inhibition on alpha-glucosidase was noncompetitive. In addition, when this fraction was orally administered to mice dosed with Acarbose (20 mg/kg), the active fraction (100, 300, 500 mg/kg) significantly suppressed increase of blood glucose levels after sucrose loading in a dose-dependent manner. These results suggest that this extract from dragon's blood exerts an anti-diabetic effect by suppressing intestinal carbohydrate absorption and thereby reducing the postprandial increase of blood glucose.
Introduction
Dragon's blood is a substance exuded by the dragon trees (Dracaena cochinchinensis) either naturally or through cuts in trunks and branches. For many years, the natural resin “dragon's blood” has been highly valued for its medical treatments [Citation1]. It has been used in antiquity widely in medicine such as an astringent in diarrhea and dysentery, in addition to an antiseptic, antiulcer, and haemostatic [Citation2]. It also plays a role to promote blood circulation [Citation3].
Diabetes mellitus is a chronic metabolic disorder characterized by high blood glucose levels [Citation4,Citation5,Citation6]. One of the therapeutic approaches for reducing postprandial hyperglycemia is to prevent absorption of carbohydrates after food intake through inhibiting alpha-glucosidase activity [Citation7,Citation8]. Therefore, alpha-glucosidase inhibitors such as acarbose, voglibose, and miglitol are widely used either alone, or in combination with insulin secretagogues in patient with type 2 diabetes [Citation9,Citation10,Citation11]. Recently, several alpha-glucosidase inhibitors have been isolated from the Eucommia Bark, Mulberry Leaves, Polygoni Cuspidati Rhizoma,Rhizoma Anemarrhenae, Rheum Palmatum L, Tea extracts and other natural sources such as (2S)-3,7-Dihydroxy-4-methoxy-8-methylflavan, (2S)-4,7-Dihydroxy-3-methoxy-8-methylflavan,(2S)-4,7-Dihydroxy-3-methoxyflavan, (2S)-4,7-Dihydroxy-8-methy-lflavan, (2S)-4,5-Dihydroxy-7-methoxy-8-methylflavan [Citation12].
The dragon's blood was reported to have strong activities against alpha-glucosidase compared to Acarbose [Citation13,Citation14]. Considering a crude material was used in Huang's study, an extraction method was developed in current study to get a fine active fraction from dragon's blood. The extractant was further tested for its inhibitory function and postprandial anti-hyperglycemic effect in vitro and in vivo, respectively.
Materials and methods
Materials and apparatus
Samples of dragon's blood (Dracaena cochinchinensis) was sold by Guangxi Traditional Chinese Medical Factory. KH2PO4, n-Butyl alcohol, NaOH, HCl, K2HPO4 and Na2CO3 used in the extraction and the activity measurement were all of analytical grade and purchased from China National Medicines Corporation Ltd. (Shanghai, China). Alpha-Glucosidase (Yeast) was from United States Biological Inc. (Swampscott, Massachusetts, USA), and 4-Nitrophenyl-α-D-glucopyranosid (PNPG) was made by Merck (Darmstadt, Germany).
In the animal experiments, KM Mice weighing 22–25 g were supplied from Experimental Animals laboratory of Fudan University. (Shanghai, China). They were housed in conventional cages with free access to water and rodent chow at 20–22°C with a 12-h light–dark cycle. All procedures involving the use of laboratory animals were in accordance with relevant guidelines.
Glucose, benzoic acid, phenol, Na2HPO4, KH2PO4, and Alloxan were used. They are also analytical grade (Shanghai, China).
Extraction of the active fraction
The dragon's blood was first extracted by petroleum ether in soxhlet extractor at 85°C for about 60 h. Then the residue was extracted by acetone also in soxhlet extractor also at 85°C for about 60 h. The same method was used to the extractant with ethyl acetate. Finally, the ethyl acetate extractant was evaporated to dryness with vacuum.
Three gram ethyl acetate extractant were first dissolved in 150 mL n-Butyl alcohol. NaOH solution (2%, 100 mL) was used to extract the ethyl acetate extractant in n-butyl alcohol in separator funnel and the NaOH layer was collected. The above step was repeated 3 times and all the NaOH layer solution was combined. After that the solution was adjusted to pH1-2 with HCl. The oily substances were collected by reduced pressure, washed to neutral with pure water, and finally desiccated.
Activity measurement
Briefly, the enzyme reaction was performed using PNP-glycoside as a substrate in phosphate salt buffer. The alpha-glucosidase inhibiting effect of the extractant was assayed according to the following procedure. Different volume of the extractant (1 mg/mL) was added into the phosphate salt buffer (pH = 6.8), after adding 2μl alpha-glucosidase (0.5 unit/μL), the mixture was incubated at 37°C for 10 min. Then 50μL PNP-glycoside was added as a substrate into the buffer, after 10-min reaction 10 mL sodium carbonate was added to terminate the reaction.
Enzymatic activity was quantified by measuring the p-nitrophenol released from PNP-glycoside at 400 nm wavelength. The activity of the dragon's blood crude material was tested as comparison.
Kinetics of enzyme inhibition
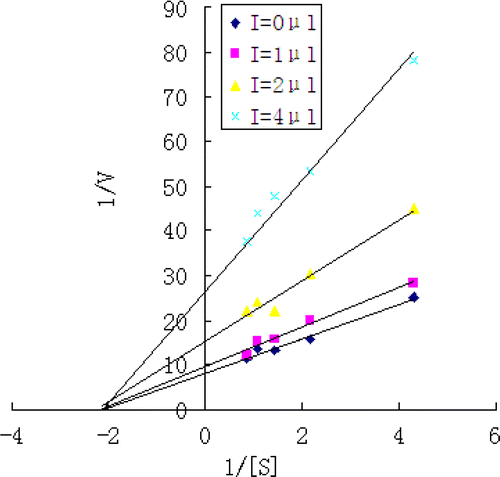
In order to examine the type of the inhibitor, alpha-glucosidase activity was measured with the increasing concentrations of PNP-glycoside (0.116 mol/L) in the absence or presence of the active fraction extracted from dragon's blood (1 mg/mL) 1μL, 2μL and 4μL. The type of the inhibitor was then determined by Lineweaver–Burk plot analysis of the data resulted from enzyme assays containing various concentration of PNP-glycoside. [Citation15]
Effect of fine extracant of the dragon's blood on diabetic mice induced by alloxan
Mice (22-25 g) were fasted for 14 h and intraperitoneal injected with 2% alloxan dissolved in the physiological salt solution. Animals with blood glucose levels higher than 11.1 mol/L were chose as diabetes models for further treatment. Those animals were divided into 5 groups randomly including a negative control group, three dose groups(100 mg/kg, 300 mg/kg, 500 mg/kg), and a positive control group. Animals were treated (gavage) with vehicle, the extractant, or acarbose once per day for 8 days. Before the last time of dosing, the animals were fast for 8 hours, and blood was collected from the ophthalmic venous sinus 2 h after the gavage. Serum was obtained after centrifuged and used for glucose level measurement. Experiments were conducted in accord with Institute procedures for Animal work.
Effect of fine extractant of the dragon's blood on pancreas islet in the blood islet of normal mice
Mice (22–25 g) were divided into five groups (13 per group), including a negative control group, three dose groups (100 mg/kg, 300 mg/kg, 500 mg/kg), and a positive control group. All the groups had been given the vehicle, active extractant, or gliquidone via gavage at a volume of 0.10 mL/10 g once per day for 14 days. Blood was collected from the ophthalmic venous sinus at the end of experiment and serum was obtained after centrifuged. The pancreas islet (INS) was determined using radiation immune method.
Statistical analysis
Results were expressed as mean ± standard error of mean (S.E.M). Statistical significances were evaluated by Student's t-test. P-values less than 0.05 were considered to be significant.
Results
The extractant increased inhibitory ability on alpha-glucosidase
As showed in , the active extractant has an IC50 of 0.152 (μg/mL), which is only half of that for dragon's blood crude material. That is to say, half of the volume of the crude material is needed for the extractant to inhibit 50% of the alpha-glucosidase activity. The extractant showed an enhanced inhibitory ability on alpha-glucosidase, and therefore, effect of the fine extractant on treating diabetes has been improved dramatically compared to dragon's blood crude material.
Tabel I. The invitro-enzyme activity of the sample and the raw material.
Kinetic analysis of alpha-glucosidase inhibition by the active extractant from dragon's blood
The inhibition mode of the active extractant against alpha-glucosidase activity was analyzed by using the data derived from enzyme assays containing various concentration of PNP-glycoside at each different concentration of the inhibitor. Double-reciprocal plots of enzyme kinetics demonstrated noncompetitive inhibition of alpha-glucosidase activity (). Secondary replot of 1/rate against [I] from a primary Lineweaver–Burk plot for the determination of Ki was showed in . The value of R2 was 0.97. The Km value was 0.464 mM and the Ki value was 1.408 mg/mL.
Effect of the fine extractant from dragon's blood on mice with diabetes induced by alloxan
The fine extraction of dragon's blood dramatically reduced the serum glucose levels in the 3 dosing groups compared to the negative control group (P < 0.01) (). In addition, glucose levels in the medium and high dosing groups were comparable to that in the positive control group in which mice were dosed with Acarbose.
Tabel II. Effect of the fine extractant from dragon's blood (Dracaena cochinchinensis) on reducing glucose levels in mice with diabetes induced by Alloxan.
Effect of the fine extractant from dragon's blood on the pancreas islet in the blood islet of normal mice
The fine extractant increased pancreas islet levels in serum in three treatment groups compared to that in the negative control group as showed in , especially in the medium and high dose group (P < 0.01), in which pancreas islet levels were comparable to that in the positive control treated with gliquidone.
Tabel III. Effect of the fine extractant from dragon's blood (Dracaena cochinchinensis) on the pancreas islet in the blood islet of normal mice.
Discussion
With the extraction method developed in the current study, an active fraction from dragon's blood was generated and displayed enhanced activities against the diabetes in in vitro and in vivo experiments.
Alloxan selectively destroyed the pancreas islet β-cells in mice and the serum glucose levels were thus increased [Citation16,Citation17]. Reduced glucose levels in mice with diabetes induced by Alloxan indicated that the fine extractant from dragon's blood can reduce the damage of Alloxan to pancreas islet β-cells or repair the damaged β-cells, and therefore weaken the symptom of diabetes.
Traditional insulin sensitizer can be transported to the pancreas after absorbed into blood, and has selective effect on the ATP—Sensitive K Channels (KATP) of pancreas islet β-cells [Citation16]. In addition to reducing the pressure of the pancreas, traditional insulin sensitizer will stimulate the pancreas islet to be released at an early time as well, so that the release of the insulin antagonist will be inhibited and internal environmental high blood glucose levels after meal can be avoided. Hence the high-density lipoprotein cholesterol and its various negative sides could be avoided. It can also stimulate acid-base dephosphorylation of the glucose transporter four (GLUT4) from the muscles and adipose tissue so that they can easily reach the cell membrane and enhance the expression of GLUT4, thus strengthen absorption of glucose directed by the pancreas islet. One of the mechanisms of dragon's blood effect on curing diabetes could be increased pancreas islet levels by the fine extractant, since pancreas islet levels in mice serum was significantly increased with the extractant treatment, especially with high concentrations, as observed in the current study.
References
- Compendiun of Materia Medica, Beijing: The Academy Press.
- PF Tu, J Tao, YQ Hu, and et al (2003). Flavones from the Wood Dracaena conchinchinensis. 1:27–29.
- Q Zhang, and H Zhu. (2004). Latest Study on the dragon's blood. Acta Academjae Medicinae CPAPF 13:69–71.
- TJ Orchard, KY Forrest, D Ellis, and DJ Becker. (1997). Cumulative glycemic exposure and microvascular complications in insulin-dependent diabetes mellitus. The glycemic threshold revisited. Arch Intern Med 157:1851–1856.
- DB Corry, and ML Tuck. (2000). Protection from vascular risk in diabetic hypertension. Curr Hypertension Rep 2:154–159.
- IM Stratton, AI Adler, HA Neil, DR Matthews, SE Manley, CA Cull, D Hadden, RC Turner, and RR Holman. (2000). Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. 321:405–412.
- LH Chen, QY Ma, and B Yang. (2005). Study on α- glucos idase inhibitor. Food Science and Technology 8:91–94.
- VV Krasikov, DV Karelov, and L Firsov. (2001). α-Glucosidases. Biochemistry (Moscow) 66:267–281.
- PS Johnston, HE Lebovitz, RF Coniff, DC Simonson, P Raskin, and CL Munera. (1998). Advantages of alpha-glucosidase inhibition as monotherapy in elderly type 2 diabetic patients. J Clin Endocrinol Metab 83:1515–1522.
- N Saito, H Sakai, S Suzuki, H Sekihara, and Y Yajima. (1998). Effect of an alpha-glucosidase inhibitor (voglibose), in combination with sulphonylureas, on glycaemic control in type 2 diabetes patients. J Internat Med Res 26:219–232.
- E Standl, HJ Baumgartl, M Fuchtenbusch, and J Stemplinger. (1997). Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diab Obes Metab 1:215–220.
- Y-J Shim, H-K Doo, S-Y Ahn, Y-S Kim, J-K Seong, I-S Park, and B-H Min. (2003). Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol 85:283–287.
- Huang MH, Yong KL. The Fingerprints Establishment of Dragon's Bloods and Their Inhibitive Activity toα-Glucosidase M.D Thesis 2002.
- Sun S. Quality control studies on traditional Chinese medicine Resina Draconis M.D Thesis 2002.
- T Matsui, T Ueda, T Oki, K Sugita, N Terahara, and K Matsumoto. (2001). alpha-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J Agricul Food Chem 49:1948–1951.
- T Miki, F Tashiro, T Iwanaga, K Nagashima, H Yoshitomi, H Aihara, Y Nitta, T Gonoi, N Inagaki, J-I Miyazaki, and S Seino. (1997). Abnormalities of pancreatic islets by targeted expression of a dominant-negative KATP channel. Proc Natl Acad Sci 94:11969–11973.
- S Seino, T Iwanaga, K Nagashima, and T Miki. (2000). Diverse roles of KATP channels learned from Kir6.2 genetically engineered mice. Diabetes 49:311–318.
![Figure 2. Secondary replot of slope vs. [I] from a primary Lineweaver–Burk plot for the determination of Ki.](/cms/asset/6d8607cc-58c0-4897-90cb-e4d41e81d39d/ienz_a_293805_f0002_b.gif)