Abstract
A series of Co(II), Ni(II) and Cu(II) complexes have been synthesized by template condensation of 2,6-diformyl-4-methylphenol and 3-substituted-4-amino-5-hydrazino-1,2,4-triazole with CoCl2·6H2O, NiCl2·6H2O and CuCl2·2H2O chlorides in 2 + 2+2 molar ratio in ethanol. These complexes were characterized by elemental analyses, magnetic susceptibility, molar conductance, spectral (IR, Uv-Vis, ESR, 1H NMR and FAB-mass), thermal, fluorescence and solid-state DC electrical conductivity studies. The observed molar conductance values indicate that they are non-electrolytes. Elemental analyses suggest the complexes to have 2:1 stoichiometry of the type [M2LX2] 2H2O (M = Co(II) & Cu(II), L = LI-LIV and X = Cl) and [Ni2LX22H2O] 2H2O. The solid state DC electrical conductivity showed that the complexes were semiconducting. All the Schiff bases and their Co(II), Ni(II) and Cu(II) complexes were evaluated for their microbiological properties and some compounds showed promising results.
Introduction
Arylhydrazone chemistry owes its origin to Emil Fischer, who found that phenyl hydrazine reacted with carbonyl compounds to form the corresponding hydrazones [Citation1]. Physiological activities of these compounds have been studied by several investigators [Citation2–4] since 1953. In recent years, nitrogen heterocyclic hydrazide and hydrazones have attracted much attention as potential biological activity and analytical reagents [Citation5]. Metal chelating properties of these hydrazones were first described by Lion and co-workers [Citation6–10] who isolated Mn(II), Co(II), Ni(II), Cu(II), Zn(II) Cd(II) and Hg(II) chelates of the simplest ligand of the type, pyridine-2-aldehyde-2-pyridyl hydrazones. Bakir and co-workers have discussed the structural aspects of hydrazones and their metal complexes in a classic article [Citation11].
Recently, a number of attempts have been made to obtain five new complexes of Cu(II), Zn(II), Fe(III), Mn(III),Co(III) and OV(IV)&(V) complexes with hydrazones [Citation12–14]. The complexes have been well characterized by elemental analyses, spectroscopic and thermal techniques.
A deep survey of literature revealed that metal chelates of o-hydroxyaraldehyde hydrazones bearing ON donor function have been extensively investigated; but those derived from heterocyclic systems particularly those containing 1,2,4-triazole moiety have received comparatively less attention. Apart from these hydrazones, metal chelates of some hydrazine derivatives derived from heterocyclic systems such as 1,2,4-triazoles and 2,6-diformyl-4-methyl phenol, the major attention being focused on those derived from 1,2,4-triazoles. This is done so, because this study is mainly pertinent to bishydrazines derived from 3-substituted-4-amino-5-hydrazino-1,2,4-triazoles and 2,6-diformyl-4-methyl phenol by template condensation.
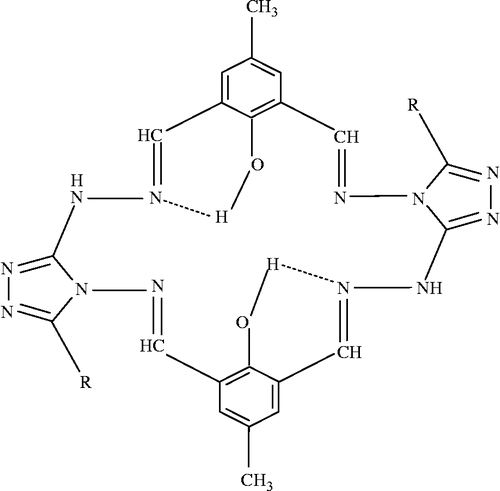
These ligands have six donor sites of NON NON sequence with varied coordinating abilities. This nature of the ligands have attracted our attention because of their biological activity, solid sate DC electrical conductivities and fluorescence properties and aroused our interest in elucidating the structure of Co(II), Ni(II) and Cu(II) complexes with these Schiff bases as there is scant information on these complexes of the following ligands ().
Materials and methods
All chemicals used were of reagent grade. 2,6-diformyl-4-methyl phenol was prepared from known methods [Citation15,Citation16]. The metal contents were estimated gravimetrically by the standard method [Citation17]. Carbon, hydrogen and nitrogen were estimated by using C, H & N analyzer. The results of elemental analyses and molar conductance values are listed in .
Table I. Elemental analyses, magnetic and molar conductance data of Co(II), Ni(II) and Cu(II) complexes of Schiff bases (H2LI- H2LIV).
Analytical and physical measurements
The IR spectra of the ligands and their Co(II), Ni(II) and Cu(II) complexes were recorded on a HITACHI-270 IR spectrophotometer in the 4000–250 cm− 1 region in KBr disc. The electronic spectra of the complexes were recorded in DMF on a VARIAN CARY 50-BIO UV-spectrophotometer in the region of 200–1100 nm. The 1H NMR spectra were recorded in DMSO-d6 on an BRUKER 300 MHz spectrometer at room temperature using TMS as an internal reference. FAB mass spectra were recorded on a JEOL SX 102/DA-6000 mass spectrometer/data system using Argon/Xenon (6 KV, 10Am) as the FAB gas. The accelerating voltage was 10 KV and the spectra were recorded at room temperature m-Nitrobenzyl alcohol was used as the matrix.
The mass spectrometer was operated in the + ve ion mode. The electrochemistry of Cu(II) complexes were recoded on CHI1110A-electrochemical (HCH Instruments) analyzer (Made in U.S.A). Thermogravimetric analyses data were measured from room temperature to 1000°C at a heating rate of 10°C/min. The data were obtained by using a PERKIN-ELMER DIAMOND TG/DTG instrument. Solid-state DC electrical conductivity of solid complexes was measured using digital micro voltmeter model DMV-001. Molar conductivity measurements were recorded on ELICO-CM-82 T Conductivity Bridge with a cell having cell constant 0.51 and magnetic moment was carried out on faraday balance.
Synthesis
Synthesis of 3-substituted-4-amino-5-hydrazino-1,2,4-triazoles
A mixture of 3-substituted-4-amino-5-mercapto-1,2,4-triazole and Hydrazine hydrate (N2H4·H2O) in 1:1 molar proportions in ethanol (50 mL) was refluxed for 4–5 h on a water bath. The reaction mixture was cooled at room temperature, within an hour the compound separated from the clear solution. It was filtered, washed and recrystallized from ethanol.
Synthesis of macrocyclic (20-membered) Schiff bases (H2LI-H2LIV)
2,6-Diformyl-4-methylphenol (2 mmol) in ethanol (20 mL) was added to a ethanolic solution of 3-substituted-4-amino-5-hydrazino-1,2,4-triazole (2 mmol, 30 mL) containing few drops of concentrated HCl. The reaction mixture was refluxed for 3 h. The mixture was cooled to room temperature and the solvent removed under reduced pressure until a solid product was formed that was washed with cold ethanol and dried under vacuum. ()
Synthesis of Co(II), Ni(II) and Cu(II) complexes (1–12)
Co(II), Ni(II) and Cu(II) complexes, were prepared adapting template method owing to the insolubility of the ligands (H2LI-H2LIV) in common organic solvents. 2,6-Diformyl-4-methylphenol (2 mmol), 3-substituted-4-amino-5-hydrazino-1,2,4-triazoles and respective metal chloride (2 mmol) in ethanol (50 mL) were refluxed for 2–3 h. The separated complexes were collected by filtration, washed with hot ethanol and dried in vacuum desiccators over P2O5.
Results and discussions
Chemistry
All the Co(II), Ni(II) and Cu(II) complexes are colored, stable in air and non-hygroscopic solids. These complexes are soluble in DMF and DMSO. The elemental analyses show that, the Co(II), Ni(II) and Cu(II) complexes have 2:1 stoicheometry of the type [M2LX2]·2H2O (M = Co(II) & Cu(II), L = LI-LIV and X = Cl) and [Ni2LX22H2O]·2H2O. The molar conductance values are too low to account for any dissociation of non-electrolytes in DMF ().
Infrared spectral studies
The important IR frequencies of the Schiff bases and their Co(II), Ni(II) and Cu(II) complexes are presented in . In the Schiff bases it is generally observed that the intramolecular H-bonded-OH occurs in the region 2700–2600 cm− 1 as a broad weak band with fine structure [Citation18].
Table II. The important infrared frequencies (in cm−1) of Schiff bases (H2LI–H2LIV) and their Co(II), Ni(II) and Cu(II) complexes.
In addition to these, a medium intensity broad band is observed around 3100 cm− 1 in these Schiff bases and this assigned to the ν(NH). It is also observed that, the medium to high intensity band around 1620 cm− 1 was assigned to ν(HC = N). That confirms the presence of the salicylidene residue [Citation19].
A medium intensity bands in the region of 1600–1590 cm− 1 are regarded as combination of C = N of triazole ring, C = C of aromatic ring and aromatic C = C stretching vibrations. A high intensity band in the region 1295–1290 cm− 1 with an additional band around 1505 cm− 1 was assigned to the phenolic ν(C–O) vibration.
The characteristic band due to ν(C = N) appears around 1605 cm− 1 for these Co(II), Ni(II) and Cu(II) complexes. The low frequency shift of ν(C = N) band suggesting that, the C = N group is coordinated to the metal ion through nitrogen and this has resulted in lowering of the bond order of carbon to nitrogen link. The band due to ν(C = N) of the heterocyclic ring for the complexes appears almost in the same region as observed in the ligands [Citation20].
A broad weak band with fine structure in the region 2700–2600 cm− 1 assigned to the H bonded-OH in the Schiff bases disappeared in the all Co(II), Ni(II) and Cu(II) complexes. The high intensity band due to phenolic C–O appeared in the region 1295–1290 cm− 1 in the Schiff bases appeared as a medium to high intensity band in the 1360–1370 cm− 1 regions in the complexes. These observations support the formation of M-O bands via deprotonation. So the H atoms of –OH groups have been replaced by the metal ion [Citation21].
Further the appearance of new bands in the 1530–1550 cm− 1 region in all the complexes suggest phenoxide bridging with metal ions.
All Co(II), Ni(II) and Cu(II) complexes exhibited a broad medium intensity bands around 3000 cm− 1 due to ν(NH) vibrations. The presence of broad stretching vibrations in the 3440–3400 cm− 1 region can be attributed to coordinated or lattice water molecules in all these complexes [Citation20].
In the light of previous assignments [Citation22,Citation23] the medium intensity bands found in the region 474–400 cm− 1 are assigned to the ν(M-N) vibrations coupled with ligand vibrations and a medium intensity band in the region 500–400 cm− 1 to the ν(M-O) vibration in view of the previous assignments [Citation24,Citation25].
Previous studies on ν(M-Cl) stretching vibrations have shown that ν(M-Cl) vibrations are sensitive to metal oxidation state and coordination number of central metal ion, and are useful in predicting stereochemistry of the complexes [Citation26,Citation27]. These bands usually appear as medium to weak intensity bands in the region 400–300 cm− 1. In view of these observations the weak intensity bands observed 340 cm− 1 are assigned to ν(M-Cl) vibrations.
1H NMR spectral studies
The 1H NMR spectra of Schiff bases (H2LI-H2LIV) have been studied. The 1H NMR spectra of Schiff bases (H2LI-H2LIV) exhibited signals around 13.78 ppm due to –NH protons [Citation25,Citation26]. Another resonance due to the azomethine protons in all the compounds appears at 10.93 ppm.
We also observed a multiplet around 7.77–8.00 ppm due to aromatic protons. All the compounds exhibit a signal around 10.19 ppm due to phenolic OH proton. Compounds exhibit a signal around 2.5 ppm due to methyl protons. All these observations provide support for the IR inferences.
Magnetic studies
The magnetic moments of the Co(II), Ni(II) and Cu(II) complexes obtained at room temperature are listed in . The Co(II) (1–4) and Ni(II) (5–8) complexes shown magnetic moment values around 4.12–4.28 and 3.16–3.26 BM respectively which are lower than the respective spin only values and indicates weak antiferromagnetic coupling interaction between the metal ions which further confirm the dinuclear nature of the complexes. The Cu(II) complexes of ligands (H2LI-H2LIV) showed a magnetic moment around 1.44 1.41 BM which is considerably lower than the spin only value for Cu(II) (9–12) complexes. The low value of the magnetic moment is attributed for the antiferromagnetic coupling interaction between two metal ions. This fact suggests the dinuclear nature of the Cu(II) complexes [Citation28].
Electronic spectral studies
The electronic spectra of Co(II) complexes exhibit absorption bands in the region 10985, 17243 and 20045 cm− 1 which can be assigned to the transitions 4A2+4E → 4B1, 4A2+4E → 4E (P) and 4A2+4E → 4A2 (P) respectively which are characteristic of square pyramidal geometry [Citation29].
The Ni(II) complexes shown three bands around 10426, 15358 and 21697 cm− 1 which are assigned to 3A2g (F) → 3T2g (F) (ν1), 3A2g (F) → 3T1g (F) (ν2) and 3A2g (F) → 3T1g (P) (ν3) transition respectively, indicating octahedral geometry [Citation29]. The parameters are listed in .
Table III. Ligand Field Parameters Of Ni(ii) Complexes Of Schiff Bases (H2li–h2liv).
The Cu(II) complexes of ligands (H2LI-H2LIV) exhibited a high intensity band at 27472 cm− 1 in the UV-region. Appearance of this band is due to π → π* transition associated with the azomethine linkage and L → M charge transfer transition. The electronic spectrum of Cu(II) complexes shown three bands at 10964, 14814 and 23148 cm− 1, these bands have been assigned to the transition 2B1 → 2A1 (ν1), 2B1 → 2B2 (ν2) and 2B1 → 2E (ν3) respectively. These transitions are characteristic of square pyramidal geometry [Citation30].
FAB-mass spectral studies
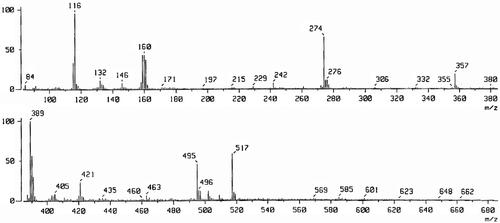
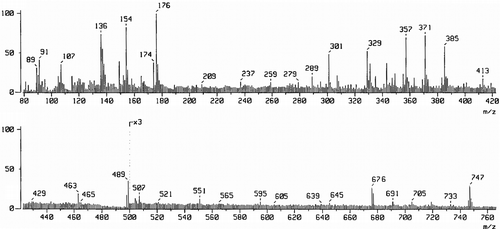
The FAB mass spectrum of Schiff base (H2LII) has been depicted in . The spectrum showed a molecular ion peak at m/z 517 that is equivalent to its molecular weight. The fragments in the spectrum leading to the formation of the species [C24H24N12O2]+.
The FAB mass spectrum of Ni(II) (5) complex of ligand (H2LI) has been depicted in . The spectrum showed a molecular ion peak M+ at m/z 747, which is equivalent to its molecular weight of the Ni(II) (5) complex. The molecular ion peak fragmentation with the loss of four water molecules, gave a peak A1 at m/z 676 due to the fragment ion [Ni2(C22H20N12O2)Cl2]+. Further, the fragments leading to the formation of the species [Ni2L]+ which under goes demetallation to form the species [L + H]+ gave fragment ion at m/z 489. All these fragmentation patterns are well observed in the FAB-mass spectrum.
These entire fragmentation patterns are well observed in the FAB mass spectrum (). It clearly indicates dinuclear nature of the complex and two Ni(II) ions are held in the macrocyclic comportment of the Schiff base (H2LI). The metal ions are bonded to two phenoxo bridges, which endogenously coordinated to the metal ions and the other coordinating sites in the ligand are the azomethine nitrogen atoms. Both phenoxide and azomethine groups surround the two metal ions, which are in close proximity with in the ligand molecule to form a square base. A chloride ion coordinates to each metal ion from opposite sides to give square pyramidal configuration to the metal ions. The FAB mass spectrum confirms the dinuclear nature of the metal complex.
ESR studies
The X-band ESR spectrum of Cu(II) (10) complex with ligand (H2LI) was recorded at room temperature using DPPH as a reference standard (). The g∥ and g⊥ values have been found to be 2.253 and 2.095 respectively. In general, dinuclear Cu(II) complexes give broad ESR peaks and the broadening is assigned to a dipolar interaction [Citation31]. The observed ESR spectrum is characteristic of square pyramidal geometry. The gav value was calculated to be 2.147. The existence of g∥ > g⊥ suggests that dx2 − y2 orbital is in the ground state and d9 configuration is (eg)4(a1g)2(b2g)2(b1g)1. The ‘g’ values are related to the axial symmetry and g∥ > g⊥ suggests square pyramidal geometry of Cu(II) complex. The axial symmetry parameter G = 2.542 which has less than 4.0 indicates considerable exchange interaction between metal ions in the solid complex which further supports the dinuclear nature of the Cu(II) complexes.
Thermal studies
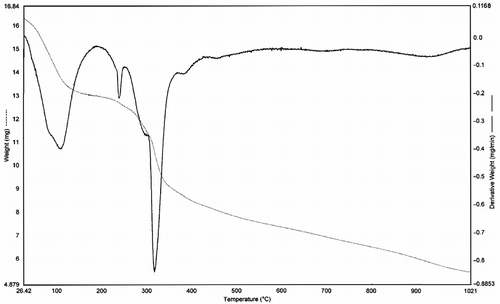
TG and DTG studies were carried out for Co(II) (2), Ni(II) (6) and Cu(II) (10) complexes at room temperature and one representative Ni(II) (6) complex has been reproduced in . These complexes decompose gradually with the formation of respective metal oxide above 600°C. The nature of proposed chemical change with the temperature range and the percentage of metal oxide obtained are given in the . The thermal decomposition of respective Co(II) (2) Ni(II) (6) and Cu(II) (10) complexes takes place in three steps as indicated by DTG peaks around 115–130, 230–260 and 300–320°C corresponding to the mass loss of two coordinated/adhered water molecules, two aldehyde moieties and two triazole moieties respectively.
Table IV. Thermogravimetric data of Co(II) (2), Ni(II) (6) and Cu(II) (10) complexes of Schiff base (H2LII).
Cyclic voltammetry
The electrochemical behavior of Cu(II) (10) complex was examined by employing glassy carbon as working electrode, Ag/AgCl as reference electrode and platinum wire as auxiliary electrode. The working media consisted of DMF and n-Bu4NClO4 as supporting electrolyte. The cyclic voltammogram of Cu(II) (10) complex in 10− 3 M solution was recorded at room temperature in the potential range − 3.0 V to +2.4 V with a scan rate 100 mV s− 1.
The complex shows a redox process corresponding to the Cu(II) → Cu(I) couple at Epa = +1.24 V and associated cathodic peak at Epc = − 0.72 V. This couple is found to be quasi-reversible as the peak separation between the anodic and cathodic potential is very high. But the ratio between the anodic and cathodic currents suggests that the process is simple one-electron transfer, quasi-reversible process [Citation32,Citation33].
On comparing the cyclic voltammograms we observed that the variation in oxidation and reduction potential may be due to distortion in the geometry of the complex which arises due to different anions coordinated to the metal ion [Citation34].
Fluorescence Studies
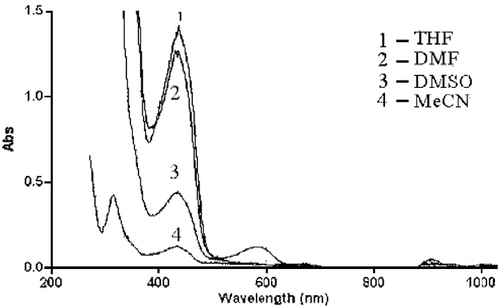
The emission spectrum of Schiff base (H2LII) was investigated in various solvents viz., DMF, DMSO, MeCN and THF.
The Schiff base (H2LII) showed a weak absorption band at 435 nm () due to 2,6-diformyl-4-methyl-phenolate ion [Citation35]. The emission band of Schiff base was observed around 560 nm in DMF, DMSO and MeCN is due to the formation of phenoxide anion and cleavage of the imine bond was observed in the Schiff base (H2LII) [Citation36]. Upon addition of aqueous alkali (2% NaOH) to all the above prepared solutions we observed the λmax of the Schiff base undergoes red shift in DMF, DMSO and MeCN solutions is due to hydrogen bond formation.
The Co(II) (2), Ni(II) (6) and Cu(II) (10) complexes were characterized by an emission band around 545 nm in DMF and DMSO and it is also observed that, the emission band of the Schiff base around 560 nm was shift towards red shift region in Co(II) (2), Ni(II) (6) and Cu(II) (10) complexes is due to the interaction of phenolic oxygen with the metal ions. We also observed complexation with fluorescence enhancement interaction in above mentioned Co(II) and Cu(II) complexes in DMF and DMSO solutions [Citation37]. Where as in Ni(II) complex (6) we could observed fluorescence quenching quite effectively [Citation38]. Magnetic perturbations, redox activity, etc., have been invoked [Citation38] in the past to rationalize fluorescence quenching by transitional metal ions. In case of Co(II) (2) and Cu(II) complex (10), obviously none of these processes are operational.
Solid-state DC electrical conductivity
The DC electrical conductivity of Cobalt(II) (2), Nickel(II) (6) and Cupper(II) (12) complexes were measured in pellet form (coated with silver paste) in the temperature from 30°C to 225°C. The pellet was dried at 100°C around 24 h in air, and then the sample was allowed to cool slowly to room temperature. Resistive behavior of these samples was studied as a function of temperature using the standard probe method.
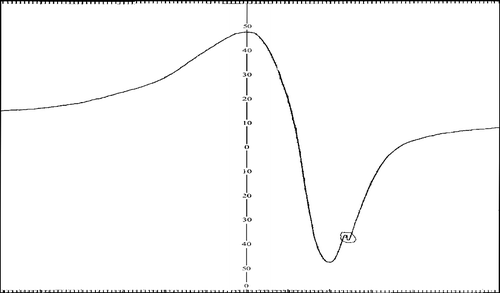
Typical plot of electrical conductivity (logσ) versus temperature (T− 1) for the above said complexes are shown that, as the temperature increases there is a gradually decreases in DC resistivity. In these complexes, the conductivity increases with increase in temperature indicating that, these complexes are semiconductors.
The increase in conductivity can be explained on the basis of clustering of ions. The formation of clusters effectively lower the concentration of stable bands and conduction will be due to hopping process. The calculated activation energy may be attributed to the interaction between the electrons of d-orbitals of a cation and the π-orbitals of the ligand. Thus, interaction will localize the π-electronic charge on the ligand molecules.
Pharmacology
In vitro antibacterial and antifungal activities
The biological activities of the newly synthesized Schiff bases (H2LI-H2LIV) and their metal complexes have been studied for their antibacterial and antifungal activities by disc diffusion method [Citation39]. The antibacterial activities were done by using gram + ve organisms (Staphylococcus aureus and Bacillus cereus) and gram − ve organisms (Pseudomonas aeruginosa and Escherichia coli). These bacterial strains were chosen as they are known as human pathogens and Aspergillus niger and Aspergillus fumigates were used for antifungal activities at 10, 25, 50 and 100 μg/mL concentrations in solvent DMF. Where DMF poured disc was used as negative control. The bacteria were subcultured in agar medium. The petri dishes were incubated for 24 h at 37°C. Standard antibacterial drug (Gentamycine) was also screened under similar conditions for comparison. The fungi were subcultured in potato dextrose agar medium. Standard antifungal drug (Fluconazole) was used for comparison. The Petri dishes were incubated for 48 h at 37°C.
The microbial results of Schiff bases and their Co(II), Ni(II) and Cu(II) complexes are systematized in and (). The Schiff bases H2LI-H2LIV show high active against B. cereus organism especially in case Schiff base (H2LI) we obtained interesting results which show high activity i.e. almost equal to the standard against B. cereus. Amongst the Co(II) complexes, (1) exhibits high active against B. cereus and others are inactive towards E. coli, P. aeruginosa and S. aureus. In case of Ni(II) (5), (6), (7) & (8) complexes show inactive towards E. coli, P. aeruginosa, B. cereus and S. aureus. Amongst the Cu(II) complexes, (10) exhibits high active against B. cereus and others are inactive towards E. coli, P. aeruginosa and S. aureus.
Table V. Bacteriological results of Schiff bases derived from 3-substituted-4-amino-5-hydrazino-1,2,4-triazoles and 2-6 diformyl-4-methyl phenol and their Co(II), Ni(II) and Cu(II) complexes.
Figure 7. (A) Antibacteriogram of Schiff bases; (B) Antifungal screening of Schiff bases; (C) Antibacteriogram of metal complexes; (D) Antifungal screening of metal complexes.
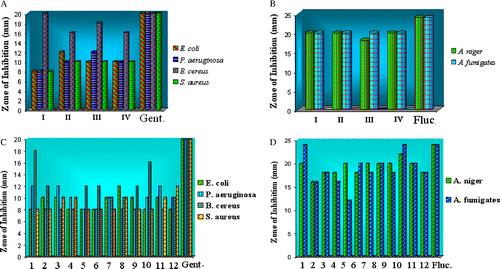
In the case of antifungal activity all the Schiff bases and their Co(II), Ni(II) and Cu(II) complexes show high activity against A. niger and A. fumigates especially in case of Co(II) (1) & Cu(II) (10) complexes we obtained interesting results that, which show high activity i.e. almost equal to the standard against A. fumigates.
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) of some selected compounds, which showed significant activity against selected bacterial and fungal species, was determined using the disc diffusion method. The MIC of these compounds varies from 10–100 μg/mL. The results are indicated that these compounds were the most active in inhabiting the growth of the tested organisms.
The biological activity of the ligands exhibited a markedly enhancement on coordination with the metal ions against all fungal strains. However, the metal complexes showed good antifungal activity against A. niger and A. fumigates. It was evident from the data that this activity significantly increased on coordination. This enhancement in the activity may be rationalized on the basis that their structures mainly possess an additional C = N bond. It has been suggested that the ligands with nitrogen and oxygen donor systems inhibit enzyme activity, since the enzymes which require these groups of their activity appear to be especially more susceptible to deactivation by metal ions on coordination. Moreover, coordination reduces the polarity [Citation40,Citation41] of the metal ion mainly because of the partial sharing of its positive charge with the donor groups [Citation42] with in the chelate ring system formed during coordination. This process, in turn, increases the lipophilic nature of the central metal atom, which favors its permeation more efficiently through the lipid layer of the micro-organism [Citation43–44] thus destroying them more aggressively.
Conclusion
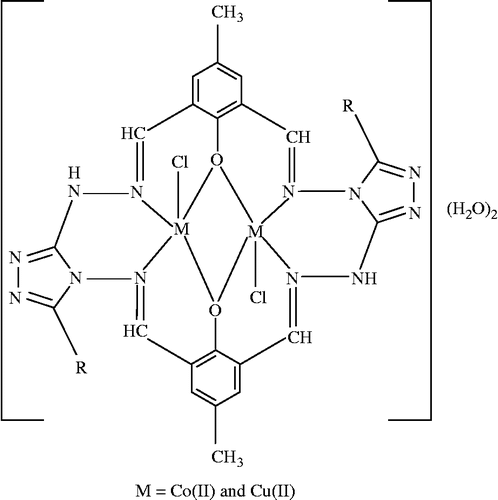
The synthesized Schiff bases (H2LI-H2LIV) acted as hexadentate ligands through the coordination of azomethine nitrogens and phenolic oxygen atoms to the metal ion. The bonding of ligands to the metal ion was confirmed by analytical, IR, 1H NMR, ESR, electronic, magnetic, FAB mass and thermal studies. All compounds were insoluble in water and decomposed at higher temperatures. All these observations put together lead us to propose the structure shown below () in which the complex has the stoichiometry of the type [M2LX2]·2H2O (M = Co(II) & Cu(II), L = LI-LIV and X = Cl) and [Ni2LX22H2O]·2H2O.
Acknowledgements
The authors are grateful to Prof. S. T. Nandibewoor, Head, Department of Chemistry, Karnatak University, Dharwad for the facilities. Thanks are also due to Prof. A. Venkataramana, Department of Material Science, Gulbarga University, Gulbarga, for the facilities to obtain solid state d.c. electrical conductivity data. Thanks are also to USIC for spectral facilities. Thanks are also due to Mr. Gururaj Rao Department of Biotechnology, Gulbarga University, Gulbarga, for the facilities to obtain the Biological results.
References
- E Fischer. (1878). Ueber die. Hydrazinverbindungen; erste abhandlung. Annalen 190:67.
- Q Albert. (1953). Nature 9:370.
- JM Price. (1961). Fed Proc 20:223.
- JM Price, RR Brown, and FR Larson. (1957). J Clin Invest 36:1600.
- M Katyal, and Y Dutt. (1975). Analytical applications of hydrazones. Talanta 52:51.
- F Lion, and KV Martin. (1958). Sexadentate chelate compounds X. J Am Chem Soc 80:3858.
- JF Geldard, and F Lions. (1962). New aromatic-type chelate compounds. J Am Chem Soc 84:2262.
- JF Geldard, and F Lions. (1963). Tridentate chelate compounds III. Inorg Chem 2:270.
- RW Green, PS Hallman, and F Lions. (1964). Multidentate ligand equilibria. I. pyridine-2-aldehyde-2-pyridylhydrazone. Inorg Chem 3:376.
- JF Geldard, and F Lions. (1965). Tridentate chelate compounds. V1.l copper(I1) complexes derived from pyridine-2-aldehyde-2′-pyridylhydrazone. Inorg Chem 4:414.
- M Bakir, AW Mark, Lawrence, and MS Wilmot. (2007). Synthesis and characterization of a cadmium-dichloro compound of N,N,O-di-2-pyridyl ketone thiophene-2-carboxylic acid hydrazone (g3-dpktch). The structure of [CdCl2(g3-pktch)]. J Coord Chem 60:2385.
- UO Zdemir, OS Senturk, S Sert, N Karacan, and F Ugur. (2006). Reaction of metal carbonyls with 2-hydroxy-1-naphthaldeyde methanesulfonyl hydrazone and characterization of the substitution products. J Coord Chem 59:1905.
- PIS Maia, VM Deon, EJ de Souza, E Garcia, and GF de Sousa. (2005). Biomimetic oxovanadium(IV) and (V) complexes with a tridentate (N,N,O)-donor hydrazonic ligand. Two X-ray crystal structure modifications of (2-acetylpyridine-benzoylhydrazonato) dioxovanadium(V). Trans Met Chem 30:404.
- B Murukan, and K Mohanan. (2007). Synthesis, characterization and antibacterial properties of some trivalent metal complexes with [(2-hydroxy-1-naphthaldehyde)-3-isatin]-bishydrazone. J Enz Inhib Med Chem 22 (1):65.
- CN Verani, E Rentschler, T Weyhermuller, E Bill, and P Chaudhuri. (2000). Exchange coupling in a bis(heterodinuclear) [CuIINiII]2 and a linear heterotrinuclear complex CoIIICuIINiII. Synthesis, structures and properties. J Chem Soc Dalton Trans 251.
- DA Denton, and H Suschitzky. (1963). Synthetic uses of polyphosphoric acid. J Chem Soc 4741.
- AI Vogel, editot. A text book of quantitative chemical analyses. 5th Edn London: Wesley Longman; (1999).
- JE Kovacic. (1967). The C = N stretching frequency in the infrared spectra of Schiff's base complexes-I. Copper complexes of Sclicylidene anilines. Spectrochim Acta 23:183.
- PG Avaji, BN Reddy, and SA Patil. (2006). Synthesis, spectral characterization, biological and fluorescence studies of lanthanum(III) complexes with 3-substituted-4-amino-5-hydrazino-1,2,4-triazole Schiff bases. Trans Met Chem 31:842.
- R Shasidhara, and TR Goudar. (2001). Synthesis of uranium(IV) complexes of Schiff bases. J Indian Chem Soc 78:360.
- R Malhotra, K Sudhir, Jyoti, HR Singal, and KS Dhindra. (2000). Synthesis, Characterization and peroxidase activity of binuclear complexes of 2,6-bis(2-hydroxybenzylideen hydrazone)-4-methyl phenol. Indian J Chem 39:421.
- CK Choudhary, RK Choudray, and LK Mishra. (2003). Complexes of Mn(III) and Co(III) with some Salicylidene-4,4-disubstituted-3-thiosemicarbazides. J Indian Chem Soc 80:693.
- BB Mohapatra, and SK Saraf. (2003). Polymetallic complexes. Part-LXXXXII. Bis-bidentate and Bis-tridentate azodye dimeric complexes of Co(II), NI(II), Cu(II), Zn(II), Cd(II) and Hg(II). J Indian Chem Soc 80:696.
- K Nakamoto, PJ Mcarthy, A Ruby, and AE Martell. (1961). Infrared spectra of metal chelate compounds. II. Infrared spectra of acetylacetonates of trivalent metals. J Am Chem Soc 83:1066.
- Parcy. (1976). Infrared spectrum of isotopically substituted cis–bis (glycino) Cu(II) monohydrate. Spectrochim Acta 31:1287.
- RJH Clark. (1965). Metal-halogen stretching frequencies in inorganic complexes. Spectrochim Acta 21:955.
- TA Khan, Shahjahan, and SA Xaidi. (1997). Synthesis, characterization of Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II) Zn(II), Hg(II), Ru(III), Rh(III), Pt(IV) and Au(III) complexes with 1-(2′-pyridyl) benzothiazole-2-thione. Indian J Chem 153.
- R Kannappan, R Mahalakshmy, TM Rajchdiran, R Venkateshan, and P Sambashiva Rao. (2003). Magnetic, catalytic, EPR and electrochemical studies on binuclear copper(II) complexes derived from 3,4-disubstituted phenol. Proc Indian Acad Sci (Chem. Sci.) 115:
- DN Satyanarayana. Electronic absortption spectroscopy and related techniques. Hyderabad: University Press; (2001).
- N Sengottuvelan, J Mohanmani, and M Kondaswamy. (2002). Synthesis of unsymmetrical compartmental oxime nickel(II) and copper(II) complexes: Spectral, electrochemical and magnetic studies. Polyhedron 21.
- MC Jain, AK Shrivastava, and PC Jain. (1977). Some tetragonally distorted copper(II) complexes of 4-benzylamidothiosemicarbazide and its thiosemicarbazone. Inorg Chim Acta 23:199.
- Z Shirin, and RN Mukherjee. (1992). Synthesis, spectra and electrochemistry of ruthenium(III) complexes with cage-like Schiff-base ligands. Polyhedron 11:2625.
- A Shyamala, and AR Chakravarty. (1993). Synthesis, structure and electrochemical properties of complexes with a (μ-oxo)bis-(μ-carboxylato) diruthenium (III) core. Polyhedron 12:1545.
- S Chandra, LK Gupta, and Sangeetika. (2004). Synthesis, physicochemical and electrochemical studies on Mn(II), Co(II), Ni(II), and Cu(II) Complexes with an N-Donor Tetradentate (N4) macrocycle ligand derived from ethyl cinnamate. Synth React Inorg Met-Org Chem 34:1591.
- S Mukarjee. (1987). Interaction of piperdine & triethylamine with 2,6-diformyl-4-methylphenol in aprotic & protic solvents: Spectrophotometric & fluorescence studies. Indian J Chem 26A:1002.
- AS Ameernisha, and PS Zacharias. (1998). Fluorescence emission in Schiff bases of 2,6-difromyl-4-methylphenol: Effect of chelation on the emission. Indian J Chem 37A:696.
- EV Akkaya, ME Huston, and AW Czarnik. (1990). Chelation-enhanced fluorescence of anthrylazamacrocycle conjugate probes in aqueous solution. J Am Chem Soc 112:3590.
- AW Varnes, RB Dodson, and EL Wehry. (1972). Interactions of transition-metal ions with photoexcited states of flavines. Fluorescence quenching studies. J Am Chem Soc 94:946.
- EO Offiong, and S Martelli. (1994). Antibacterial activity of metal complexes of benzil and benzoin thiosemicarbazone IL. Farmaco 49:513.
- CJ Balhausen. An introduction to ligand field. New York: McGraw Hill; (1962).
- ABP Lever. Inorganic electronic spectroscopy. Elsevier; Amsterdam: (1984).
- ZH Chohan, A Scozzafava, and CT Supuran. (2002). Unsymmetrical 1,1′-disubstituted Ferrocenes: Synthesis of Co(ii), Cu(ii), Ni(ii) and Zn(ii) Chelates of Ferrocenyl -1-thiadiazolo-1′-tetrazole, -1-thiadiazolo-1′-triazole and -1-tetrazolo-1′-triazole with Antimicrobial Properties. J Enz Inhib Med Chem 17 (14):261.
- ZH Chohan, KM Khan, and CT Supuran. (2004). Synthesis of antibacterial and antifungal cobalt(II), copper(II), nickel(II) and zinc(II) complexes with bis-(1,1′-disubstituted ferrocenyl)thiocarbohydrazone and bis-(1,1′-disubstituted ferrocenyl)carbohydrazone. Appl Organomet Chem 18:305.
- ZH Chohan. (2004). Synthesis and biological properties of Cu(II) complexes with 1,1′-disubstituted ferrocenes. Synth React Inorg Met-Org Chem 34:833.