Abstract
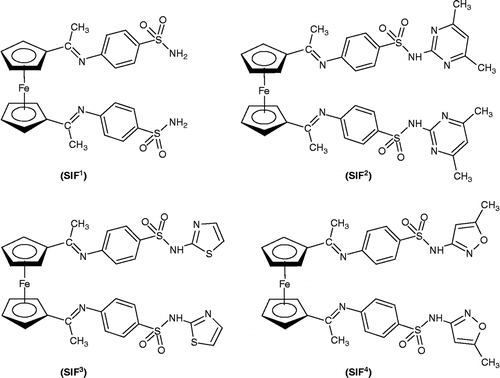
Sulfonamides incorporated ferrocene (SIF) have been synthesized by the condensation reaction of sulfonamides (sulfanilamide, sulfathiazole or sulfamethaxazole) with 1,1′-diacetylferrocene. The synthesized compounds (SIF1–SIF4) have been characterized by their physical, spectral and analytical properties and have been screened for their in vitro antibacterial properties against pathogenic bacterial strains e.g., Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis Staphylococcus aureus and Salmonella typhi and for antifungal activity against Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glaberata using Agar-well diffusion method. Most of the compounds showed good antibacterial activity whereas, all the compounds exhibited significant antifungal activity. Brine shrimp bioassay was also carried out for in vitro cytotoxic properties against Artemia salina.
Introduction
The development and “designing” of new drugs has revolutionized the practice of medicine. In designing new drugs, it is significant to know about the nature of disease or the infectious process. Drug resistance too, has become globally a striking medical and clinical problem. It is therefore, essential specifically and selectively designing such drugs targeting the progression process where the resistance mechanism emerges. The relationship [Citation1,Citation2] between biological activity and metal ions compiles one potential possibility of designing novel therapeutic methodologies in addressing probable mechanism of resistance. In this regard, a number of studies have highlighted the use of ferrocene and its derivatives for the design of biologically active compounds Citation3–5. Some reports have signified [Citation6,Citation7] that if certain group in some antibiotics is replaced by ferrocene the antibacterial properties are appreciably improved. Also, many activities such as antibacterial Citation8–10, antitumor [Citation11,Citation12], anti-carbonic anhydrase [Citation13,Citation14], hypoglycaemic [Citation15], anti-thyroid [Citation16] and protease inhibitor [Citation17] are related to the potential use and application of sulfonamide drugs. Combining together the chemistry of ferrocene and sulfonamides attracted the attention of the author in designing and introducing new biologically active organometallic compounds that may prove to fight more aggressively against certain bacteria and also resistant ones. Acylferrocene undergoes a condensation reaction with amines to form Schiff bases. Making a successful attempt underlying this transformation, we wish to report some sulfonamide incorporated ferrocenes (SIF1–SIF4) () along with their structural elucidation. In order to emulate the biological activity of proteins and enzymes, the prepared sulfonamide incorporated ferrocenes have been screened for their antibacterial activity against pathogenic bacterial strains such as Escherichia coli, Pseudomonas aeruginosa, Bacillus subtillis, Staphylococcus aureus and Salmonella typhi and, for antifungal activity against Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glaberata using the agar-well diffusion method [Citation18,Citation19].
Material and methods
All solvents used were of Analytical grade. 1,1′-Diacetylferrocene and sulfonamides were obtained from Aldrich. All metals were used as their chloride salts. IR and NMR spectra were recorded on Perkin Elmer 283B and 300 MHz Varian XL-300 instruments. UV Visible spectra were obtained on a Baush and Lomb spectronic 1001. Conductances of the metal complexes were determined in DMF using a YSI-32 model conductometer. Magnetic measurements were done on solid complexes using the Gouy method. Melting points were recorded on a Gallenkamp apparatus and are uncorrected.
General procedure for the preparation of SIF1–SIF4
A solution of respective sulfonamide (0.01 mol) in ethanol (20 mL) was added drop-wise to a solution of 1,1′-diacetylferrocene (1.35 g, 0.005 mol) in ethanol (15 mL). The reaction mixture was heated under reflux for 8 h, cooled to room temperature, filtered and refrigerated overnight. The precipitated product thus formed during refrigeration was filtered, washed with ethanol (2 × 3 mL) followed by diethyl ether. After drying, the product was recrystallized from hot ethanol to obtain dark brown solid, which was dried under vacuum. The purity of the product was confirmed by TLC [plastic sheets of a silica gel F254 with 0.2 mm layer thickness].
1,1′-Sulfanilamide incorporated ferrocene (SIF1)
IR (KBr, cm− 1): 3390 (NH2), 1625 (C = N), 1345, 1110 (S = O), 952 (S–N), 833 (C–S); 1H NMR (DMSO-d6, δ, ppm): 2.5 (s, 6H, CH3), 4.4–4.6 (m, 4H, ferrocenyl), 4.9–5.2 (m, 4H, ferrocenyl), 7.4–7.6 (m, 4H, Ph), 7.8–8.0 (m, 4H, Ph), 11.3 (s, 2H, SO2NH2); 13C NMR (δ, ppm): 15.7 (CH3), 68.6, 69.6, 83.6 (ferrocenyl), 138.2, 128.6, 122.6, 156.4 (Ph), 161.0 (C = N, azomethine).
1,1′-Sulfamethazine incorporated ferrocene (SIF2)
IR (KBr, cm− 1): 3265 (NH), 1625 (C = N), 1345, 1110 (S = O), 952 (S–N), 833 (C–S), 1580 (pyrimidinyl, C = N); 1H NMR (DMSO-d6, δ, ppm): 2.5 (s, 6H, CH3), 2.3 (s, 6H, CH3-pyrimidinyl), 4.4–4.6 (m, 4H, ferrocenyl), 4.9–5.2 (m, 4H, ferrocenyl), 8.2 (s, 1H, pyrimidinyl), 7.3–7.5 (m, 4H, Ph), 7.6–7.8 (m, 4H, Ph), 11.4 (s, 1H, SO2NH); 13C NMR (δ, ppm): 25.1 (CH3-pyrimidinyl), 15.7 (CH3), 68.6, 69.2, 83.8 (ferrocenyl), 165.2, 103.0, 168.5 (pyrimidinyl), 138.2, 128.6, 122.6, 156.4 (Ph), 161.8 (C = N, azomethine).
1,1′-Sulfathiazole incorporated ferrocene (SIF3)
IR (KBr, cm− 1): 3260 (NH), 1620 (C = N), 1345, 1110 (S = O), 952 (S–N), 833 (C–S), 1610 (thiazolyl, C = N); 1H NMR (DMSO-d6, δ, ppm): 2.5 (s, 6H, CH3), 4.4–4.6 (m, 4H, ferrocenyl), 4.9–5.2 (m, 4H, ferrocenyl), 7.4–7.6 (m, 4H, Ph), 7.7–7.9 (m, 4H, Ph), 8.1 (d, 1H, thiazolyl), 8.3 (d, 1H, thiazolyl), 11.3 (s, 1H, SO2NH); 13C NMR (δ, ppm): 15.7 (CH3), 68.6, 69.2, 83.8 (ferrocenyl), 108.0, 137.8, 171.7 (thiazolyl), 138.2, 128.6, 122.6, 156.4 (Ph), 161.6 (C = N, azomethine).
1,1′- Sulfamethaxazole incorporated ferrocene (SIF4)
IR (KBr, cm− 1): 3265 (NH), 1620 (C = N), 1345, 1110 (S = O), 952 (S–N), 833 (C–S), 1615 (isoxazolyl, C = N); 1H NMR (DMSO-d6, δ, ppm): 2.5 (s, 6H, CH3), 2.3 (s, 3H, CH3- isoxazolyl), 4.4–4.6 (m, 4H, ferrocenyl), 4.9–5.2 (m, 4H, ferrocenyl), 7.4–7.6 (m, 4H, Ph), 7.8–8.0 (m, 4H, Ph), 8.2 (s, 1H, isoxazolyl), 11.3 (s, 1H, SO2NH); 13C NMR (δ, ppm): 15.7 (CH3), 68.6, 69.2, 83.8 (ferrocenyl), 12.8 (CH3-isoxazolyl), 159.6, 95.0, 150.3 (isoxazolyl), 138.2, 128.6, 122.6, 156.4 (Ph), 162.3 (C = N, azomethine).
Biological properties
Antibacterial bioassay (in vitro)
All the synthesized compounds (SIF1–SIF4) were screened in vitro for their antibacterial activity against E. coli, P. aeruginosa, B. subtillis, S. aureus and S. typhi bacterial strains by the agar-well diffusion method [Citation18,Citation19]. The wells (6 mm in diameter) were dug in the media with the help of a sterile metallic borer with centers at least 24 mm apart. Two to eight hours old bacterial inocula containing approximately 104–106 colony-forming units (CFU/mL) were spread on the surface of the nutrient agar with the help of a sterile cotton swab. The recommended concentration of the test sample (1 mg/mL in DMSO) was introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, imipenum, served as negative and positive controls, respectively. The plates were incubated immediately at 37°C for 24 hours. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). In order to clarify any participating role of DMSO in the biological screening, separate studies were carried out with the solutions alone of DMSO and they showed no activity against any bacterial strains. Also, the metal salts used for complexation were evaluated for antibacterial activity against the same bacterial strains under the same conditions but none of them showed any significant and/or moderate activity, only insignificant activity was observed.
Antifungal activity (in vitro)
All compounds were studied against six fungal cultures for Antifungal activities. Sabouraud dextrose agar (oxoid, Hampshire, England) was seeded with 105 (cfu) mL− 1 fungal spore suspensions and transferred to petri plates. Discs soaked in 20 ml (200 μg/mL in DMSO) of all compounds were placed at different positions on the agar surface. The plates were incubated at 32°C for seven days. The results were recorded [Citation20] by measuring the diameter of zones showing complete inhibition (mm). Miconazole and amphotericin B were used as standard drugs for this bioassay.
Minimum inhibitory concentration (MIC)
Compounds containing high antibacterial activity (over 80%) were selected for minimum inhibitory concentration (MIC) studies. The minimum inhibitory concentration was determined using the disc diffusion technique by preparing discs containing 10, 25, 50 and 100 μg/mL of the compounds and applying the protocol [Citation21,Citation22].
Cytotoxicity (in vitro)
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm), filled with artificial seawater, which was prepared with commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the matter compartment was opened to ordinary light. After two days nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 mL of DMF. From this stock solutions 500, 50 and 5 μg/mL were transferred to 9 vials (three for each dilutions were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 mL of DMF only. The solvent was allowed to evaporate overnight. After two days, when shrimp larvae were ready, 1 mL of sea water and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with sea water to 5 mL per vial. After 24 h the number of survivors was counted. Data were analyzed by Finney computer program to determine the LD50 values [Citation23,Citation24].
Results and discussion
Chemistry
The compounds (SIF1–SIF4) were prepared by refluxing ethanol solution of respective sulfonamide e.g., sulphanilamide, sulfamethazine, sulfathiazole, sulfamethaxazole, with 1,1′-diacetylferrocene () in 2:1 (sulfonamide: diacetylferrocene) molar ratio. All the products were obtained as solids and their purities were checked by TLC (eluent = n-hexane/chloroform/ethylacetate, 1/2/1 v/v). All the synthesized compounds were characterized by spectroscopic techniques (IR, 1H NMR & 13C NMR) given in experimental part and by their elemental analyses. Colour, m.p, yield (%) and CHN analyses for compounds (SIF1–SIF4), are given in . Microcrystalline powder of these compounds could only be obtained, which were impossible to be used for X-ray structural determinations.
Tabel I. Physical measurements and analytical data of sulfonamide incorporated ferrocene (SIF1–SIF4).
IR spectra
In the IR spectra of compounds (SIF1–SIF4), a sharp band at 1620–1625 cm− 1 is assigned [Citation25] to the stretching of ν(C = N). Presence of this band is an evidence of the formation of condensation product. νasymm(SO2) and νsymm(SO2) vibrations appeared at 1345 and 1110 cm− 1, respectively [Citation26,Citation27]. The IR spectra of SIF1 showed peak at 3390 cm− 1, due to NH2 stretchings, whereas the IR spectra of SIF2–SIF4 exhibited peak at 3260–3265 cm− 1, due to NH stretching vibrations. In addition, the spectra of SIF2–SIF4 showed bands resulting from the pyrimidinyl (C = N), thiazolyl (C = N), isoxazolyl (C = N) stretchings at 1580, 1610 and 1615 cm− 1, respectively. The presence of all these frequencies were supportive [Citation28,Citation29] of the formation of compounds (SIF1–SIF4).
1H NMR spectra
The 1H NMR of all the compounds (SIF1–SIF4) were taken in DMSO-d6. The data reported along with the possible assignments in experimental part show that SIF1–SIF4 displays all expected signals at δ 2.5, 4.4–4.6 and 4.9–5.2 which are assigned to CH3 and the ferrocenyl moities, respectively 1H NMR spectra of the compounds showed multiplet at δ 7.3–7.6 for the phenyl ring system. The SO2NH2 and SO2NH- protons in all cases appeared as a singlet in the region of δ 11.3–11.4, respectively. The 1H-NMR spectrum of SIF2 displayed pyrimidinyl proton as singlet at δ 8.2. In case of SIF3, thiazolyl protons appeared as doublet at δ 8.0 and 8.3. In case of compound SIF4, the isoxazolyl proton appeared as a singlet at δ 8.2. The spectra of all the four compounds displayed methyl protons peaks as singlets at δ 2.5. Furthermore, the number of protons calculated from the integration curves [Citation30] and those obtained from the values of the expected CHN analyses agree well with each other.
13C NMR spectra
The 13C NMR of all the compounds (SIF1–SIF4) were also taken in DMSO-d6. The spectra of all the four compounds displayed azomethine (CH = N) peak at δ 161.0 which further supported the results obtained in IR, 1H NMR, hence formation of condensation product. 13C NMR spectra of the compounds (SIF1–SIF4) displayed signals of CH3 and ferrocenyl at δ 15.7, 68.6, 69.2, 83.8 respectively. The phenyl carbons appeared at δ 138.2, δ 128.6, δ 122.6 and δ 156.4, respectively in all the compounds whereas the CH3-pyrimidinyl and other pyrimidinyl carbons appeared at δ 25.1, 165.2, 103.0, 168.5 respectively in SIF2. In SIF3, the thiazolyl carbons appeared at δ 108.0, 137.8 and 171.7 respectively. In SIF4, CH3-isoxazolyl and other isoxazolyl carbons appeared at δ 12.8, 159.6, 95.0 and 150.3 respectively. Furthermore, the number of carbons calculated from the spectra [Citation31] and those obtained from the values of the expected CHN analyses compromise well with each other.
Biological activity
Antibacterial bioassay (in vitro)
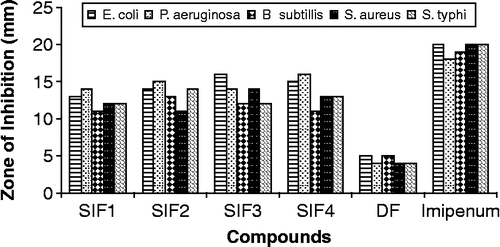
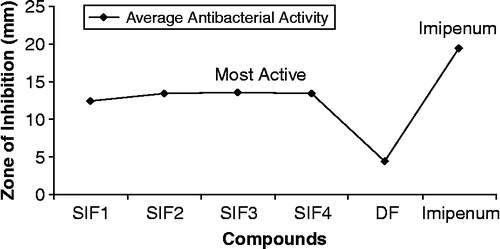
All compounds were tested against bacterial strains such as E. coli, P. aeruginosa, B. subtillis, S. aureus and S. typhi () according to the literature protocol [Citation18,Citation19]. The results were compared with those of the standard drug imipenum (). All compounds (SIF1–SIF4) showed moderate to significant activity against different bacterial strains except the activity of all compounds against B. subtillis where all compounds showed moderate activity. All compounds showed significant activity against E. coli and P. aeruginosa however, SIF3 and SIF4 were shown to be the most active one ().
Tabel II. Antibacterial activity data of (SIF1–SIF4).
Antifungal bioassay (in vitro)
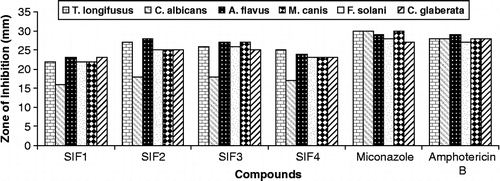
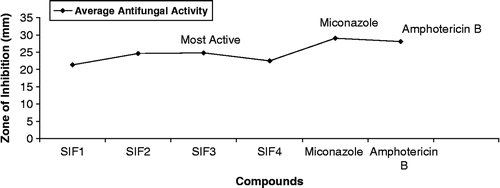
The antifungal screening of all compounds was carried out against T. longifusus, C. albicans, A. flavus, M. canis, F. solani and C. glaberata fungal strains according to the literature protocol [Citation20]. All synthesized compounds showed good antifungal activity against different fungal strains. All compounds (SIF1–SIF4) showed significant antifungal activity against all the fungal strains except C. albicans where moderate activity was observed. The results of inhibition were compared () with the results of inhibition of standard drugs miconazole and amphotericin B (). SIF3 was found to be the most antifungal compound ().
Tabel III. Antifungal Activity Data Of (Sif1–sif4).
Minimum inhibitory concentration (MIC) for antibacterial activity
The preliminary antibacterial screening showed that SIF2, SIF3, SIF4 were the most active ones (above 80%), SIF2 against P. aeruginosa, SIF3 against E. coli and SIF4 against P. aeruginosa. These compounds were therefore, selected for antibacterial minimum inhibitory concentration (MIC) studies ().
Tabel IV. Minimum inhibitory concentration (M) of the selected compounds against selected bacteria.
Cytotoxic bioassay (in vitro)
All the synthesized compounds were screened for their cytotoxicity (brine shrimp bioassay) using the protocol of Meyer et al. [Citation23]. From the data recorded in , it is evident that all the compounds displayed potent cytotoxic activity against Artemia salina. SIF1 showed activity (LD50 = 1.253 M), SIF2 showed activity (LD50 = 0.794 M), SIF3 showed activity (LD50 = 0.799 M) and SIF4 showed activity (LD50 = 0.824 M) in the present series of compounds.
Table V. Brine shrimp bioassay data of (SIF1–SIF4).
Conclusion
The results of this investigation support the suggested structures of the sulphonamide incorporated ferrocenes. It has been suggested [Citation32,Citation33] that some functional groups such as azomethine or heteroaromatics present in these compounds display extensive biological activities that may be responsible for the increase of hydrophobic character and liposolubility of the molecules. It ultimately enhances activity of the compounds and biological utilization ratio.
Acknowledgements
The author gratefully acknowledges HEJ research Institute of Chemistry, University of Karachi for the help in undertaking NMR spectra and biological assay.
References
- Z Xiaoxian, L Youngmin, N Fajun, and M Yongxiang. (1992). Ferrocenyl thiosemicarbazone and its transition metal complexes. Polyhedron 11:447.
- SP Singh, and NB Singh. (1990). Studies on tetrametallic complexes of ferrocene. Polyhedron 9:557.
- ZH Chohan, and CT Supuran. (2005). Organometallic compounds with biologically active molecules: In-vitro antibacterial and antifungal activity of some 1,10-(dicarbohydrazono)ferrocenes and their Co (II), Cu (II), Ni (II) and Zn (II) complexes. Appl Organomet Chem 19:1207.
- ZH Chohan. (2004). Synthesis and biological properties of Cu(II) complexes with 1,10-disubstituted ferrocenes. Synth React Inorg Met-Org Chem 34:833.
- SR Patil, UN Kantak, and DN Sen. (1983). New tin(IV) and organotin(IV) chelates of ferrocenyl hydrazones. Inorg Chim Acta 68:1.
- EI Edwards, R Epton, and G Marr. (1975). Organometallic derivatives of penicillins and cephalosporins a new class of semi-synthetic antibiotics. J Organomet Chem 85:C-23.
- BW Rockett, and G Marr. (1976). Ferrocene: Annual survey covering the year 1975. J Organomet Chem 123:205.
- GL Mandell, and WA Petri. In: JG Hardman, LE Limbird, PB Molinoff, RW Ruddon, and AG Gilman, editors. Pharmacological basis of therapeutics. 9th ed. New York: McGraw-Hill; (1966). p 1057–1072.
- TH Maren. (1976). Relations between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol 16:309–327.
- G Domagk. (1935). Chemotherapy of bacterial infections. Deut Med Wochensch 61:250.
- T Owa, and T Nagasu. (2000). Novel sulfonamide derivatives for the treatment of cancer. Exp Opin Ther Pat 10:1725–1740.
- AE Boyd. (1988). Sulfonylurea receptors, ion channels, and fruit flies. Diabetes 37:847–850.
- I Nishimori, D Vullo, A Innocenti, A Scozzafava, A Mastrolorenz, and CT Supuran. (2005). Carbonic anhydrase inhibitors: Inhibition of the transmembrane isozyme XIV with sulfonamides. Bioorg Med Chem Lett 15:3828–3833.
- A Scozzafava, F Briganti, G Mincione, L Menabuoni, F Mincione, and CT Supuran. (1999). Carbonic anhydrase inhibitors: Synthesis of water-soluble, aminoacyl/dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 42:3690–3700.
- CW Thornber. (1979). Isosterism and molecular modification in drug design. Chem Soc Rev 8:563–580.
- RC Ogden, and CW Flexner. Protease inhibitors in AIDS therapy. New York, USA, New York, USA: Marcel Dekker; (2001).
- CT Supuran, A Scozzafava, and A Mastrolorenzo. (2000). Bacterial proteases: Current theraputic use and future prospects for the development of new antibiotics. Exp Opin Therap Pat 111:221–259.
- AU Rahman, MI Choudhary, and WJ Thomsen. Bioassay techniques for drug development., Vol. 16 The Netherlands: Harwood Academic Publishers; (2001).
- KM Khan, ZS Saify, AK Zeeshan, M Ahmed, M Saeed, M Schick, HJ Kohlbau, and W Voelter. (2000). Arzneim-Forsch/Drug Res 50:915.
- JL McLaughlin, C-J Chang, and DL Smith. In: Atta-ur-Rahman. editor. Studies in natural products chemistry, “Bentch-Top” bioassays for the discovery of bioactive natural products: An update, structure and chemistry (part-B)., Vol. 9. The Netherlands: Elsevier Science Publishers; (1991). p 383.
- Atta-ur-Rahman, MI Choudhary, and WJ Thomsen. Bioassay techniques for drug development. The Netherlands: Harwood Academic Publishers; (2001). p 22.
- AW Bauer, WM Kirby, JC Sherris, and M Turck. (1966). Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493.
- BN Meyer, NR Ferrigni, JE Putnam, LB Jacobsen, DE Nichols, and JL McLaughlin. (1982). Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica 45:31.
- DJ Finney. Probit analysis. 3rd ed. Cambridge: Cambridge University Press; (1971).
- LJ Bellamy. The infrared spectra of complex molecules. New York: John Wiley; (1971).
- JR Ferrero. Low-frequency vibrations of inorganic and coordination compounds. New York: John Wiley; (1971).
- GR Burns. (1968). Metal complexes of thiocarbohydrazide. Inorg Chem 7:277.
- RC Maurya, and P Patel. (1999). Synthesis, magnetic and special studies of some novel metal complexes of Cu (II), Ni (II), Co (II), Zn (II), Nd (III), Th (IV), and UO2 (VI) with schiff bases derived from sulfa drugs, viz., sulfanilamide/sulfamerazine and o-vanillin. Spectr Lett 32:213.
- K Nakamoto. Infrared spectra of inorganic and coordination compounds 2nd ed.. New York: Wiley Interscience; (1970).
- DJ Pasto. Organic structure determination. London: Prentice Hall International; (1969).
- WW Simmons. The Sadtler handbook of proton NMRspectra. Sadtler Research Laboratories, Inc, (1978).
- L Puccetti, G Fosolis, V Daniela, ZH Chohan, S Andrea, and CT Supuran. Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff's bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes in-vitro antibacterial, antifungal and cytotoxic properties of sulfonamide-derived Schiff's bases and their metal complexes. Bioorg Med Chem Lett (2005); 15:3096.
- ZH Chohan, AU Shaikh, MM Naseer, and CT Supuran. (2006). In-vitro antibacterial, antifungal and cytotoxic properties of metal-based furanyl derived sulfonamides. J Enzy Inhib Med Chem 21:771.