Abstract
A new series of 4-phenyldiazenyl 2-(phenylimino methyl) phenols were synthesized by the condensation of 5-[(2-chloro phenyl) diazenyl] 2-hydroxybenzaldehyde with different substituted aromatic amines and sulphonamides. All the synthesized compounds were screened in-vitro for their antibacterial activity against different human pathogens viz: B. anthracis, E.coli, S. aureus, S. typhimurium, and P. aeruginosa using disk diffusion assay. All the compounds exhibited considerable inhibition against the bacteria tested.
Introduction
In all over the world, population is exposed to the burden of fatal microbial diseases. Due to acquire bacterial resistant and toxicity of drugs have highlighted the urgent need to discover new antibiotics, preferably those affordable to developing countries where infectious diseases are predominant. For the treatment of microbial infections an urgent need to develop new drugs either by synthesis of analogues, modifications in existing compounds or searching novel structure, that the concerned organisms has never been presented with before, [Citation1].
It is evident that in azomethine derivatives the C = N linkage is an essential structural requirement for biological activity. These compounds are readily hydrolyzed under acidic conditions leading to active aldehydes which can act as alkylating agents [Citation2]. Besides, several azomethines have been reported to possess remarkable antibacterial [Citation3–9], antifungal [Citation10–14], anticancer [Citation15–19], antioxidant [Citation20], antimycobacterial [Citation21], antitumor [Citation22–24], antiviral [Citation25], antiHIV [Citation26–28] herbicidal [Citation29] and diuretic activities [Citation30]. The azomethine derivatives and their complexes derived from o-formylphenoxyacetic acid with aminothiazoles, a number of aminobenzene derivatives, and some heterocyclic & aliphatic amines have revealed biological significance such as antimetabolites of pyridoxal phosphate [Citation31], bacteriostatic activity [Citation32] and chorismate synthase inhibition [Citation33]. Many attempts have been made to synthesize, characterize and to study structure-activity relationship (SAR) of schiff bases [Citation34–37]. In view of conclusions drawn from our previous work [Citation38–42] and looking to the antimicrobial efficacy of –SO2NH2, –SO2NHC (NH) NH2, –Cl and –OCH3 moieties attached to aryl ring it seems logical and attractive to combine all these moieties together in a parent molecule. This study was aimed at exploring the potential antibacterial activity resulting from the combination of pharmacophores in one structure. The results of this study may be useful to researchers attempting to gain more insight into the antibacterial activity of azomethine derivatives. In present piece of research work we have selected substituted aromatic amines having above mentioned moieties, for the synthesis of azo salicylaldehyde and finally converted it into imine by condensing with appropriate substituted aromatic amines. Synthesized compounds were characterized by elemental analysis, IR, 1H NMR & mass spectral analysis and evaluated for their antimicrobial efficacy.
Materials and methods
Chemistry
All the melting points were determined in open glass capillary and are uncorrected. The purity of compounds was ascertained by TLC on silica gel plates and spots were visualized using iodine vapors, purified by recrystallisation and column chromatography. Elemental analysis was carried out on Carlo Erba 1108 analyzer. The IR spectra were recorded on Perkin Elmer spectrophotometer, 1H NMR spectra were recorded on Varian EM-390 MHz NMR spectrometer in DMSO d6 using TMS as internal reference and chemical shift values were expressed in ppm δ Bruker DRX 300 (300 MHz, FT NMR); MS–FAB: Jeol–SX 102 Mass spectrometer.
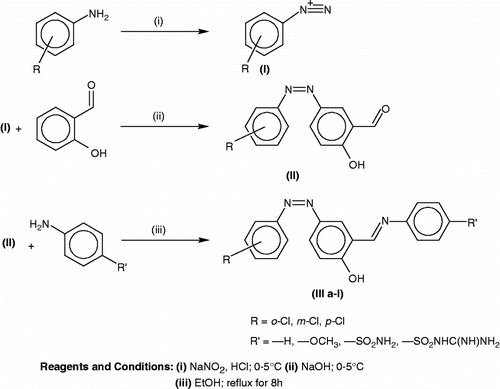
Our general synthetic route leading to new imines involved the preparation of suitably substituted aromatic azoaldehyde and subsequent coupling reaction with suitable aromatic amine resulting different aromatic imines is shown in Scheme .
General procedure for synthesis of compounds
2-hydroxy-5-[phenyldiazenyl] benzaldehyde (I)
Aniline (3.72 mL) was dissolved in aqueous hydrochloric acid (28 mL, 6 N) and mechanically stirred at 0–5°C. The cold solution of sodium nitrite (5 g in 10 mL water) was added to constantly stirred reaction mixture drop by drop.The diazotized solution was immediately added in small portions in salicylaldehyde (5 mL dissolved in 40 mL 6 N NaOH), during constant stirring at 0–5°C.The stirring was continued for 4h. The solid obtained was filtered under suction washed with cold water and recrystallised from glacial acetic acid.
4- [(-2-chlorophenyl) diazenyl] -2-[(4-substituted phenyl)imino]methyl phenol (III)
A mixture of appropriate, 2-hydroxy-5-[phenyldiazenyl] benzaldehyde (I) (1.30 g) and the suitable aniline (II) (0.46 g) were refluxed for 8 h in DMF (30 mL). The mixture was then cooled in ice bath and the product separated was repeatedly washed with water followed by ethanol and recrystallised from diethyl ether.
III(a)
Recrystallization from diethyl ether: Yield 56.2.%; m.p. 208°C; IR (KBr) cm1 3400 cm− 1 (O–H), 3250 cm− 1(N–H),1620(CH = N), 1573(N = N) 1310, 1115(S–O), 745(C–Cl); 1HNMR (DMSO d6)δ, ppm: 9.3 (s, 1H, OH), 8.3 (s, 1H, CH = N), 7.3–7.6 (m, 11H, Ar–H), 6.8 (s, 1H, SO2NH), 5.7(s, 2H, NH2); Anal. Calcd. For C20H17N6O3SCl: C 52.57, H 3.75, N 18.39, Found C 52.53, H3.70, N18.33%; MS-FAB: (m/z) 456(M+), 458(M + 2).
III(b)
Recrystallization from diethyl ether: Yield 83.6%; m.p. 180°C; IR (KBr) cm1 3420 cm− 1(O–H), 3310 cm− 1 (N–H), 1630 (CH = N), 1560(N = N) 1300, 1105(S–O), 735(C–Cl). 1HNMR (DMSO d6)δ, ppm: 9.2(s, 1H, OH), 8.1 (s, 1H, CH = N), 7.2–7.7 (m, 11H, Ar–H), 6.3 (s, 1H, SO2NH) Anal. Calcd. For C19H15N4O3SCl: C 55.01, H 3.64, N 13.50 Found C54.95, H3.60, N13.45%; MS-FAB: (m/z) 414(M+), 416(M + 2).
III(c)
Recrystallization from diethyl ether: Yield 71.4%; m.p. 184°C; IR (KBr) cm13410 cm− 1 (O–H), 1617(CH = N), 1595(N = N), 715(C–Cl). 1HNMR (DMSO d6)δ, ppm: 9.4 (s, 1H, OH), 8.3 (s, 1H, CH = N), 7.0–7.6 (m, 11H, Ar–H), 3.5 (3H, m, OCH3). Anal. Calcd. For C20H16N3O2Cl C 65.67, H 4.41, N 11.49 Found C 64.61, H 4.36, N11.45%; MS-FAB: (m/z) 365(M+), 367(M + 2).
III(d)
Recrystallization from diethyl ether: Yield 89.8%; m.p. 202°C; IR (KBr) cm1 3422 cm− 1 (O–H), 1620 (CH = N), 1573 (N = N), 735(C–Cl). 1H NMR (DMSOd6)δ, ppm: 9.3 (s, 1H, OH), 8.5 (s, 1H, CH = N), 7.3–7.6 (m, 11H, Ar–H). Anal. Calcd. For C19H14N3OCl: C 67.96, H 4.20, N 12.51 Found C67.90, H 4.15, N 12.46%; C19H14N3OCl; MS-FAB: (m/z) 335(M+), 337(M + 2).
III(e)
Recrystallization from diethyl ether: Yield 57%; m.p. 206°C; IR (KBr) cm1 3430 cm− 1 (O–H), 3250 cm− 1(N–H), 1625(CH = N), 1570(N = N) 1315, 1115(S–O), 745(C–Cl); 1HNMR (DMSO d6)δ, ppm: 9.4 (s, 1H, OH), 8.2 (s, 1H, CH = N), 7.3–7.6 (m, 11H, Ar–H), 6.8 (s, 1H, SO2NH), 5.7(s, 2H, NH2); Anal. Calcd. For C20H17N6O3SCl: C 52.57, H 3.75, N 18.39, Found C 52.53, H3.70, N18.33%; MS-FAB: (m/z) 456(M+), 458(M + 2).
III(f)
Recrystallization from diethyl ether: Yield 80.6%; m.p. 201°C; IR (KBr) cm1 3400 cm− 1(O–H), 3320 cm− 1 (N–H), 1620 (CH = N), 1560(N = N) 1310, 1105(S–O), 735(C–Cl). 1HNMR (DMSO d6)δ, ppm: 9.2(s, 1H, OH), 8.1 (s, 1H, CH = N), 7.2–7.7 (m, 11H, Ar–H), 6.3 (s, 1H, SO2NH) Anal. Calcd. For C19H15N4O3SCl: C 55.01, H 3.64, N 13.50 Found C54.95, H3.60, N13.45%; MS-FAB: (m/z) 414(M+), 416(M + 2).
III(g)
Recrystallization from diethyl ether: Yield 75.4%; m.p. 188°C; IR (KBr) cm13450 cm− 1 (O–H), 1627(CH = N), 1595(N = N), 725(C–Cl). 1HNMR (DMSO d6)δ, ppm: 9.4 (s, 1H, OH), 8.3 (s, 1H, CH = N), 7.0–7.6 (m, 11H, Ar–H), 3.5 (3H, m, OCH3). Anal. Calcd. For C20H16N3O2Cl C 65.67, H 4.41, N 11.49 Found C 64.61, H 4.36, N11.45%; MS-FAB: (m/z) 365(M+), 367(M + 2).
III(h)
Recrystallization from diethyl ether: Yield 82.8%; m.p. 196°C; IR (KBr) cm1 3422 cm− 1 (O–H), 1620 (CH = N), 1573 (N = N), 730(C–Cl). 1H NMR (DMSOd6)δ, ppm: 9.3 (s, 1H, OH), 8.5 (s, 1H, CH = N), 7.3–7.6 (m, 11H, Ar–H). Anal. Calcd. For C19H14N3OCl: C 67.96, H 4.20, N 12.51 Found C67.90, H 4.15, N 12.46%; C19H14N3OCl; MS-FAB: (m/z) 335(M+), 337(M + 2).
III(i)
Recrystallization from diethyl ether: Yield 59.2.%; m.p. 217°C; IR (KBr) cm1 3400 cm− 1 (O–H), 3250 cm− 1(N–H), 1620(CH = N), 1583(N = N) 1310, 1115(S–O), 745(C–Cl); 1HNMR (DMSO d6)δ, ppm: 9.3 (s, 1H, OH), 8.3 (s, 1H, CH = N), 7.3–7.6 (m, 11H, Ar–H), 6.8 (s, 1H, SO2NH), 5.7(s, 2H, NH2); Anal. Calcd. For C20H17N6O3SCl: C 52.57, H 3.75, N 18.39, Found C 52.53, H3.70, N18.33%; MS-FAB: (m/z) 456(M+), 458(M + 2).
III(j)
Recrystallization from diethyl ether: Yield 81.6%; m.p. 213°C; IR (KBr) cm1 3420 cm− 1(O–H), 3310 cm− 1 (N–H), 1630 (CH = N), 1570(N = N) 1300, 1105(S–O), 738(C–Cl). 1HNMR (DMSO d6)δ, ppm: 9.2(s, 1H, OH), 8.1 (s, 1H, CH = N), 7.2–7.7 (m, 11H, Ar–H), 6.3 (s, 1H, SO2NH) Anal. Calcd. For C19H15N4O3SCl: C 55.01, H 3.64, N 13.50 Found C54.95, H3.60, N13.45%; MS-FAB: (m/z) 414(M+), 416(M + 2).
III(k)
Recrystallization from diethyl ether: Yield 73.4%; m.p. 184°C; IR (KBr) cm13410 cm− 1 (O–H), 1617(CH = N), 1590(N = N), 715(C–Cl). 1HNMR (DMSO d6)δ, ppm: 9.4 (s, 1H, OH), 8.3 (s, 1H, CH = N), 7.0–7.6 (m, 11H, Ar–H), 3.5 (3H, m, OCH3). Anal. Calcd. For C20H16N3O2Cl C 65.67, H 4.41, N 11.49 Found C 64.61, H 4.36, N11.45%; MS-FAB: (m/z) 365(M+), 367(M + 2).
III(l)
Recrystallization from diethyl ether: Yield 84.8%; m.p. 154°C; IR (KBr) cm1 3422 cm− 1 (O–H), 1620 (CH = N), 1583 (N = N), 735(C–Cl). 1H NMR (DMSOd6)δ, ppm: 9.3 (s, 1H, OH), 8.5 (s, 1H, CH = N), 7.3–7.6 (m, 11H, Ar–H). Anal. Calcd. For C19H14N3OCl: C 67.96, H 4.20, N 12.51 Found C67.90, H 4.15, N 12.46%; C19H14N3OCl; MS-FAB: (m/z) 335(M+), 337(M + 2).
Microbiology
All the compounds were screened for their in vitro antimicrobial activity at Birla Institute of Medical Research and College of Life Sciences, Gwalior against 24 h old cultures of bacterial and fungal pathogens. Screening facilities (including pathogens, media and instruments) required, were provided by the same institute. Antimicrobial activity was determined against Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Bacillus anthracis, and P.aeruginosa, bacterial strains using disc diffusion assay. For this sterile filter paper disc (6 mm) impregnated with fixed doses (250, 500, 1000 μg/mL) of synthesized compounds under investigation were placed upon the seeded petri dishes. Similar disc were prepared for the standard drugs, tetracycline and solvent control, dimethyl formamide.The plates were allowed to stay for 24 h at 37°C for bacterial strains. The zone of inhibition, observed around the disc after incubation was measured. The compounds exhibited promising activities at 1000μg/disc concentration, are presented in .
Table I. In vitro antibacterial activity of the newly synthesized compounds IIIa-l (1000μg /disc).
Results and discussion
In the present work, 12 new compounds were synthesized. The synthetic route for the compounds is outlined in Scheme . For the synthesis of the title compounds, azosalicylaldehyde required as starting material, was prepared for the first time by the diazotization of aniline and coupling with salicylaldehyde. The reaction of equimolar quantities of these azosalicylaldehyde (I) with respective aromatic amines in ethanol resulted in the formation of the title compounds (IIIa-l). The structures of the obtained compounds were elucidated by spectral data. In the IR spectra, some significant stretching bands due (CH = N) and (N = N) were at 1615 cm− 1and 1580 cm− 1 respectively. In the 1H-NMR spectra, all compounds (IIIa-l) were characterized by the presence of the imino protons (CH = N) at 8.1–8.5 ppm as a singlet. Mass spectra MS (FAB) of compounds showed M+ and M + 2 peaks in agreement with their molecular formula. All compounds gave satisfactory elemental analysis.
The antibacterial activities of the synthesized compounds were screened in-vitro using B.anthracis, S.aureus, E.coli, S.typhimurium and P.aeruginosa at three different concentrations i.e., 250, 500 and 1000 μg/disc. The results of the biological evaluation, expressed as a zone of inhibition and percentage inhibition of the growth pathogens, are summarized in , and compare with standard drug tetracycline. Inspite of all the tested compounds proved to be moderate active against bacterial pathogens, can be used as lead compounds. The activity was affected by substituents on the rings. Thus, compound IIIa which including p- Cl and –SO2NHC(NH)NH2 groups showed the highest inhibition (77.50%). Other compounds showed varying degrees of inhibition between 00.00–67%. The MIC values against the test bacterial pathogens were determined at different concentration i.e. from 1000μg/mL to 15.62 μg/mL by serial dilution tube technique. For the most potent compounds IIIa, IIIb, IIIe and tetracycline control the MIC values are 250, 500, 500 and 125μg/mL respectively against the most inhibited bacterial pathogen S.aureus.
Acknowledgements
Sincere thanks are due to the Dean, BIMR College of Life Science Gwalior, for providing antibacterial screening facilities and to the UGC New Delhi, for financial assistance.
References
- S Harbart, W Albrich, DA Goldman, and Huebner. (2001). Control of multiply resistant cocci: Do international comparisons help?. J Lancet Infect Dis 1:251–261.
- WCJ Ross, and GP Warwick. (1956). Aryl (2-haloalkyl) amines. XVIII. The rates of reduction of substituted N, N-bis(2-chloroethyl)-4-phenylazoaniline by stannous chloride, hydrazine, and the xanthine oxidase-xanthine system. J Chem Soc 1724–1732.
- SV More, DV Dongarkhadekar, RN Chavan, WN Jadhav, SR Bhusare, and RP Pawar. (2002). Synthesis and antibacterial activity of new Schiff bases, 4-thiazolidinones and 2-azetidinones. J Indian Chem Soc 79:768–769.
- AK Bhendkar, K Vijay, and AW Raut. (2004). Synthesis of some novel Schiff bases of 2- aminopyrimidine and their antimicrobial activity. Acta Ciencia Indica Chem 30:29–32.
- YK Vaghasiya, RS Nair, M Baluja, and SS Chanda. (2004). Synthesis, structural determination and antibacterial activity of compounds derived from vanillin and 4-aminoantipyrine. J Serb Chem Soc 69:991–998.
- K Vashi, and HB Naik. (2004). Synthesis of novel Schiff base and azetidinone derivatives and their antibacterial activity. Eur J Chem 1:272–276.
- RC Rhodes, H Hall, SR Beesley, JE Jenkins, DC Collins, and P Zheng. (1995). Therapeutic Potentiation of the immune system by costimulatory Schiff base-forming drugs. Nature 377:71–75.
- PG More, RB Bhalvankar, and SC Pattar. (2001). Synthesis and biological Synthesis and biological activity of Schiff bases of aminothiazoles. J Indian Chem Soc 78:474–475.
- AH El-Masry, HH Fahmy, and SHA Abdelwahed. (2000). Synthesis and antimicrobial activity of some new Benzimidazole derivatives. Molecules 5:1429–1438.
- MA Basecr, VD Jadhav, RM Phule, YA Archana, and YB Vibhute. (2000). Synthesis and antimicrobial activity of some new Schiff bases. Orient J Chem 16:553–556.
- HM Safwat, FA Ragab, NM Eid, and GM Abdel. (1988). Synthesis, anti-tumor and antimicrobial activities of 3-chloro-9-(p-N-substituted sulfamoyl phenylaminoethylene)acridines. Egypt J Pharm Sci 29:99–110.
- R Mtrei, M Yadawe, and SA Patil. (1996). Synthesis of biologically active p-bis (amino-5-mercapto-1, 2, 4-triazol-3-yl) benzene and its Schiff base: A new class of bis-triazole. Orient J Chem 12:101–102.
- ME Hossain, MN Allam, J Begum, MA Akbar, MN Uddin, FE Smith, and RC Hynes. (1996). The preparation, characterization, crystal structure and biological activities of some Cu (II) complexes of the 2-benzoyl pyridine Schiff bases of S-methyl-and S-benzyldithiocarbazate. Inorg Chim Acta 249:207–213.
- I Zawadowska. (1963). Synthesis of phenoxyacetic and phenoxypropionic acids with aldehyde, acetyl, and carboxy groups in the nucleus, and their activity against human pathogenic fungi. Acta Polon Pharm 20:25–30.
- KP Sharma, VS Jolly, and P Phatak. (1998). Schiff bases and their derivatives as potential anticancer agents. Ultra Scient Phys Sci 10:263–266.
- VE Kuz'min, AG Artemenko, RN Lozytska, AS Fedtchouk, VP Lozitsky, EN Muratov, and AK Mescheriakov. (2005). Investigation of anticancer activity of macrocyclic Schiff bases by means of 4D-QSAR based on simplex representation of molecular structure. SAR QSAR Environ Res 16:219–230.
- MS Shingare, and DB Ingle. (1976). Synthesis of pyrimidine Schiff bases as anticancer agents. J Indian Chem Soc 53:1036–1037.
- DR Shkawat, SS Sabnis, and CV Deliwala. (1973). Potential anticancer agents, Schiff bases from p-(3-azaspiro [5, 5] undec-3-yl) benzaldehydes. Bull Haffkine Inst 1:35–39.
- SB Desai, PB Desai, and KR Desai. (2001). Synthesis of some Schiff bases, thiazollidones, and azetidinones derived from 2,6-diaminobenzo[1,2-d:4,5-d]bisthiazole and their anticancer activities. Hererocycl Commun 7:83–90.
- Z Guo, R Xing, S Li, H Yu, P Wang, and P Li. (2005). The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bio Med Chem Lett 15:4600–4603.
- J Potole, D Shignapurker, and S Pandhye. (2006). Schiff base conjugates of p-aminosalicylic acid as antimycobacterial agents. Bio Med Chem Lett 16:1514–1517.
- EM Hodnett, and PD Mooney. (1970). Antitumor activities of some Schiff bases. J Med Chem 13:786–788.
- EM Hodnett, and WJ Dunn. (1970). Structure-antitumor activity correlation of some Schiff bases. J Med Chem 13:768–770.
- X Yang, P Xu, Q Gao, and M Tan. (2002). Synthesis, characterization, and antitumor activity of some trivalent lanthanide complexes with 2-formylphenoxyacetic acid thiosemicarbazone. Synth React Inorg Met-Org Chem 32:59–68.
- HA Al-Khamees, SM Bayomi, HA Kandil, and C Thahir. (1990). Synthesis and pharmacological screening of a new series of 3-(4-anti-pyryl)-2-arylthiazolidin-4-ones. Eur. J Med Chem 25:103–106.
- SN Pandeya, D Shriram, E De Clercqu, C Pannecouque, and M Wtvrouw. (1998). Anti-HIV activity of some Mannich bases of isatin derivatives. Ind J Pharm Sci 60:207–212.
- SN Pandeya, D Sriram, G Nath, and E De Clercq. (1999). Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methyl mercapto quinazolin -4(3H)- one. Pharm Act Helv 74:11–17.
- SN Pandeya, D Shriram, G Nath, and E De Clercqu. (1999). Synthesis, antibacterial, antifungal and anti HIV activity of schiff and mannich bases of isatin with N-[6-Chlorobenzothiol-2yl] Thiosemicarbazide. Indian J Pharma Sci 61:358–361.
- AK Halve, and S Samadhiya. (2001). Synthetic utility of schiff bases as potential herbicidal agent. Orient J Chem 17:119–122.
- CT Supuran, M Barboiu, C Luca, E Pop, ME Brewster, and A Dinculescu. (1996). Carbonic anhydrase activators. Part 14. Syntheses of mono and bis pyridinium salt derivatives of 2-amino-5-(2-aminoethyl)- and 2-amino-5-(3-aminopropyl)-1,3,4-thiadiazole and their interaction with isoenzyme II. Eur J Med Chem 31:597–606.
- TL Hullar, and DL Failla. (1969). Pyridoxal phosphate. II. Benzene analogs. 2-Formylphenoxyacetic acids as potential antimetabolites of pyridoxal phosphate. J Med Chem 12:420–424.
- BC Ma, XM Ma, LR Yan, and D Yang. (2004). Synthesis, and bacteriostatic activity of 2-(carboxymethoxy) benzaldehyde benzoyl hydrazone and its rare earth complexes. Xingyong-Huaxue 21:841–843.
- MG Thomas, C Lawson, NM Allanson, BW Leslie, JR Bottomley, A McBride, and OA Olusanya. (2003). A series of 2(Z)-2-Benzylidene-6,7-dihydroxybenzofuran-3[2H]-ones as inhibitors of chorismate synthase. Bioorg Med Chem Lett 13:423–426.
- GS Huang, YM Liang, XL Wu, WM Liu, and YX Ma. (2003). Some ferrocenyl Schiff bases with nonlinear optical properties. Appl Organometal Chem 17:706–710.
- M Curini, F Epifano, F Maltese, and MC Marcotullio. (2002). Novel chiral Schiff base ligands from amino acid amides and salicylaldehyde. Tetrahedron Lett 43:3821–3823.
- LDS Yadav, BS Yadav, and VK Rai. (2004). A novel salicylaldehyde based mineral supported expedient synthesis of benzoxazinone nucleosides. Tetrahedron Lett 45:5351–5353.
- LX Zhang, Y Liu, LH Cia, YJ Hu, J Yin, and PZ Hu. (2006). Inhibitory study of some novel Schiff base derivatives on Staphylococcus aureus by microcalorimetry. Thermochim Acta 440:51–56.
- AK Halve, R Dubey, and D Bhadauria. (2007). N/C-4 substituted azetidin-2-ones: Synthesis and preliminary evaluation as new class of Antimicrobial agents. Bioorg Med Chem Lett 17 (2):341–345.
- AK Halve, R Dubey, D Bhadauria, B Bhaskar, and R Bhadauria. (2006). Synthesis, antimicrobial screening and structure activity relationship of some novel 2-hydroxy-5-(nitro substituted phenyl azo) benzylidene anilines. Indian J Pharm Sci 68:510–514.
- AK Halve, P Gour, R Dubey, D Bhadauria, and B Bhaskar. (2005). Synthesis and antimicrobial screening of 2′- hydroxy-5′-(phenylazo)- N-(1″,3″-diketophenylamine)-3-chloro azetidine-2-ones. Indian J Chem 44(B):2163.
- AK Halve, R Dubey, D Bhadauria, B Bhaskar, and P Gour. (2006). Synthesis of some new azetidin-2-ones as potential antimicrobial agents. J Indian Chem Soc 83:386.
- AK Halve, B Bhaskar, V Sharma, R Bhadauria, A Kankoriya, A Soni, and K Tiwari. (2008). Synthesis and in vitro antimicrobial studies of some new 3-[phenyldiazenyl] benzaldehyde N-phenyl thiosemicarbazones. J Enz Inhib Med Chem 23:77–81.