Abstract
The leaves of Saussurea triangulata (Compositae) have been eaten with rice as a wrapping vegetable for preventing neuro-aging. However, the components responsible for the neuroprotective effects of S. triangulata still remain unidentified. In the process of investigating the neuroprotective activity of S. triangulata, we found that a methanol extract of S. triangulata exhibited significant protection against glutamate-induced toxicity in primary cultured rat cortical cells. Three quinic acid derivatives were isolated from the n-BuOH fraction of S. triangulata. Among these three quinic acid derivatives, methyl 5-caffeoylquinic acid (3) exhibited significant neuroprotective activities against glutamate-induced toxicity exhibiting cell viability of about 50%, at concentrations ranging from 0.1 μM to 10 μM. Therefore, the neuroprotective effect of S. triangulata might be due to the inhibition of glutamate-induced toxicity by the quinic acid derivatives from S. triangulata.
Introduction
Alzheimer's disease (AD) is a progressive, neurodegenerative disorder associated with a global impairment of higher mental function, and presenting an impairment of memory as the cardinal symptom [Citation1] and a major cause of morbidity and disability in the adult population. Glutamate is one of the principal excitatory neurotransmitters in the brain, and its interactions with specific neurocyte membrane receptors are responsible for many neurological functions, including cognition, memory, movement, and sensation [Citation2]. Excitatory neurotransmitters also play an important role in the developmental plasticity of synaptic connections in the nervous system. However, in a variety of pathologic conditions, including stroke and various neurodegenerative disorders, such as Alzheimer's disease (AD), excessive activation of glutamate receptors may mediate neuronal injury or death. Glutamate is involved in the formation of pathological hallmarks of AD including senile plaques (SP) and neurofibrillary tangles (NFTs). However, the mechanisms involved with these effects are not very clear. [Citation3]. Thus, neuroprotection against glutamate-induced toxicity has been a therapeutic strategy for preventing and/or treating both acute and chronic forms of neurodegeneration [Citation4].
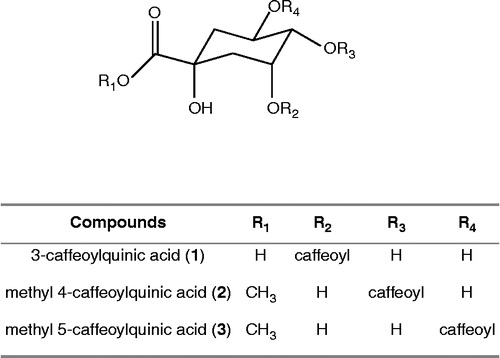
The aerial parts of Saussurea triangulata trautv. et meyer. (Compositea) have been used in the Korea folk medicine “Dum-Bul-Chi”, which is used for the treatment of inflammation, hypertension and hepatitis in Korea, Japan and China. The leaves of S. triangulata have been eaten with rice as a wrapping vegetable for preventing neuro-aging [Citation5]. Yang et al. reported the isolation of several sesquiterpene glycoside from the aerial parts of S. triangulata. [Citation6]. However, the components responsible for the neuroprotective effects of S. triangulata still remain unidentified. In the context of our natural product chemistry program dealing with the development of new potent neuroprotection agents, we have examined the isolated of major compounds as leads for novel glutamate-induced toxicity inhibitors. As part of our continued study of the neuroprotective effects of S. triangulata, we have now isolated single compounds (3-caffeoylquinic acid (1), methyl 4-caffeoylquinic acid (2), methyl 5-caffeoylquinic acid (3)) from the methanol extract of S. triangulata and examined their inhibitory effects on glutamate-induced toxicity in primary cultured rat cortical cells.
Material and methods
General
The melting points were determined on a Gallenkamp melting point apparatus and are uncorrected. The optical rotations were determined using a Jasco P-1020 polarimeter (Jasco Co., Japan). UV: UV-2200 UV–VIS recording spectrophotometer (Shimadzu, Japan); IR: Jasco Report-100 spectrophotometer; NMR: Bruker AMX 400 spectrometer (Bruker, USA), the chemical shifts being represented as ppm with tetramethylsilane as an internal standard; GC-MS (HP 5890 series II plus GC, HP 5972 series Mass Selective Detector, Column: HP-1MS); column chromatography: silica gel 60 (70 ∼ 230 and 230 ∼ 400 mesh, Merck), Sephadex LH-20 (Pharmacia, Sweden) and YMC-GEL ODS-A (12 nm, S-75 mm, YMC); TLC: pre-coated silica gel 60 F254. Dizocilpine maleate(MK-801), DL-2-amino-5-phosphonovaleric acid(APV) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNOX) were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Plant Material
S. triangulata was collected in the O-Dae mountains, in South Korea, in July 2000, and verified by Ph.D. T.-J. Kim of the Plant Taxonomy Lab of KRIBB (Dae Jeon in South Korea). A voucher specimen (SKKU-2000-8-021) was deposited in the herbarium of the Pharmacognosy Laboratory at Sung Kyun Kwan University (Kyunggi Do in South Korea)
Plant material extraction and compounds isolation from S. triangulata
The crushed dried S. triangulata (500 g) were extracted with MeOH five times under reflux for 6 h yielding 101 g of a dark blue solid extract, 100 g of which was suspended in MeOH (2 L) and precipitated by adding a 5-fold volume of acetone. The supernatant was concentrated in vacuo to yield a blue gum, which was suspended in H2O (1 L) and defatted with Et2O. The aqueous layer was extracted with aqueous saturated n-BuOH. The resulting n-BuOH solution was concentrated in vacuo to yield the n-BuOH fraction (11 g). A portion of the n-BuOH fraction (10 g) was suspended in water (300 ml), the soluble fraction was subjected to a macroreticular resin column chromatography and eluted first using water for desugarizing and decoloring, then with an EtOH-water gradient. The various eluted fractions were combined and concentrated in vacuo until the smell of EtOH had disappeared, then the concentrated solution was freezed to dryness to yield the total powder (6.3 g). The above-mentioned total lyophilized powder (5 g) was chromatographed on a silica gel column and eluted using a CHCl3-MeOH-water elution system (60:30:5, v/v/v, lower layer) to yield 10 subfractions (St-B-1 ∼10); these fractions were further separated by repetitive silica gel, Sephadex LH-20, RP-18 Lobar®-A chromatography. The major subfraction St-B-3 (125.0 mg) was purified on a RP-18 Lobar®-A column (CH3CN(2):H2O(5)) to yield compounds 1 (15.0 mg), 2 (7.0 mg) and 3 (16.0 mg). The structures of these compounds were identified by comparing their physicochemical and spectroscopic data with previous reported results.
Cortical cell culture and Cell viability assessment
Primary cultures of mixed cortical cells containing both neurons and glia were prepared from 17 ∼ 19-day-old fetal rats (Sprague-Dawley) as described previously [Citation7]. Cultures were allowed to mature for at least 2 weeks before being used for experiments. Test fraction and compounds were dissolved in DMSO (final concentration in culture, 0.1%). Cortical cell cultures were washed with DMEM and incubated with test compounds for 1 h. The cultures were then exposed to 100 μM glutamate and maintained for 24 h. After the incubation, the cultures were assess(Compositae)ed for the extent of neuronal damage by measuring the efflux of LDH (lactic dehydrogenase) which reflects the integrity of cellular membrane.
Statistical Analysis
The results are expressed as means ± standard errors (S.E.). The data were statistically analyzed by one way ANOVA. Differences with P < 0.05 were considered significant.
Results and discussion
In the present experiments, we found that a defatted aqueous extract of S. triangulata might inhibit glutamate-induced toxicity in primary cultured rat cortical cells. In order to clarify the neuroprotective components of S. triangulata, as part of a continued study of neuroprotection effects of S. triangulata, activity-guided isolation was performed to seek active fractions and components. After solvent fractionation, we compared the inhibiting effects of various fractions on neuroprotective activity and the defatted extracts of S. triangulata was found to inhibit the this activity in a dose-dependence manner in the assay system using glutamate-induced toxicity in primary cultured rat cortical cells. n-BuOH souble fractions were found to have significant inhibitory activity on neuroprotection, while the n-BuOH insouble fractions have no such activity towards glutamate-induced toxicity in primary cultured rat cortical cells. The above-mentioned results suggested that the neuroprotective actions of the defatted aqueous extracts of S. triangulata may be attributed in part to its polar component. To clarify the active substances of S. triangulata, we examined the effects of the major secondary metabolites from S. triangulata, the polar component fractions, on neuroprotective activity. Activity-guided isolation was further performed to yield single compounds. Three known quinic acid derivatives were isolated from butanol fraction by repeated column chromatography. These compounds () were identified as 3-caffeoylquinic acid (1), methyl 4-caffeoylquinic acid (2), and methyl 5-caffeoylquinic acid (3) respectively, by comparing physicochemical and spectroscopic data with literature values [Citation8, Citation9]. To investigate and compare neuroprotective activities of quinic acid derivatives isolated from S. triangulata, the activities of 1, 2, 3 were evaluated in glutamate-injured primary cultured rat cortical cells at concentrations ranging from 0.1 to 10 μM (). It is notable that the neuroprotective activities of both 1 and 2 were comparable to those of MK-801, APV and CNOX, all not effective. Additionally, among the tested three quinic acid derivatives, only 3 was effective. 3 also significantly attenuated glutamate-induced toxicity exhibiting cell viability of 50–60% at the concentrations ranging from 0.1 to 10 μM. Although more extensive structure-activity some study might be needed, these results indicate that substitution of a caffeoyl-group at R4 of quinic acid increases the neuroprotective activity. These findings about S. triangulata containing neuroprotective activities may provide some ideas for the development of agents to ameliorate the cellular dysfunction due to glutamate-induced toxicity. The aim of future study will be to isolate larger quantities of 3 from the whole parts of S. triangulata and determine this compound's mode of action against neuroprotective activity.
Table I. Neuroprotective effect of compounds from S. triangulata against glutamate-induced toxicity in primary cultured rat cortical cellsa.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
This work was supported by the MRC program of MOST/KOSEF (grant #: R13-2005-013-13 01000-0, South Korea, Won-Hwan Park and Hyung-In Moon equally contributed to this work.
References
- R Wang, and XC Tang. (2005). Neuroprotective effects of huperzine A. NeuroSignals. 14:71–82.
- NJ Sucher, M Awobuluyi, YB Choi, and SA Lipton. (1996). NMDA receptors from genes to channels. Trends Pharmacol Sci 17:348–355.
- R Cacabelos, M Takeda, and B Winblad. (1999). The glutamatergic system and neurodegeneration in dementia: Preventive strategies in Alzheimer's disease. Int J Geriatr Psychiatry 14:3–47.
- DG Trist. (2000). Excitatory aminoacid agonists and antagonists: Pharmacology and therapeutic applications. Pharm Acta Helv 74:221–229.
- KR Park. (1990). Han-Kuk-Yak-Young-Sik-Mul-Ja-Won-Bun-Ryu. RuralDevelopment Administration. Sang Rok Sa co, Suwon 223.
- MC Yang, SM Kim, KH Lee, KH Kim, and KR Lee. (2007). A new sesquiterpene glycoside from the aerial parts of Saussurea triangulata. Molecules 12:2270–2276.
- SY Kang, KY Lee, SH Sung, and YC Kim. (2005). Four new neuroprotective dihydropyranocoumarins from Angelica gigas. J Nat Prod 68:56–59.
- N Nakatani, S Kayano, H Kikuzaki, K Sumino, K Katagiri, and T Mitani. (2000). Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J Agric Food Chem 48:5512–5516.
- G Flamini, E Antognoli, and I Morelli. (2001). Two flavonoids and other compounds from the aerial parts of Centaurea bracteata from Italy. Phytochemistry 57:559–564.