Abstract
The reaction of cobalt(II) chloride with a new class of thiosemicarbazones viz; cis-3,7-dimethyl-2,6-octadienthiosemicarbazone(CDOTSC; L1H) and 3,7-dimethyl-6-octenethiosemicarbazone (DOTSC; L2H) and N-phthaloyl derivative of DL-glycine(A1H), L-alanine(A2H) or L-valine(A3H) in 1:1:1 molar ratio in dry refluxing ethanol have been studied. All the isolated complexes have the general composition [Co(L)(A)]. Tentative structures are proposed for these complexes based upon elemental analysis, electrical conductances, magnetic moment, molecular weight determination and spectral (IR, electronic) studies.The ligands and Co(II) complexes have been tested for their antibacterial and antifungal activities against three bacterial strains S. aureus, B. subtilis, E. coli and two fungal strains F. moniliformae and M. phaseolina. Attempts have been made to establish a correlation between the antibacterial and antifungal activity and the structures of products.
Introduction
The synthesis and biological activities of complexes of thiosemicarbazones continue to attract attention, since Domgk first reported the anticancer activity of thiosemicarbazones [Citation1–3]. The potential biological activity of compounds containing N and S may be responsible for this increased interest. The transition metal complexes with N, O and S donor ligands have remarkable potential for inhibiting the growth of various pathogenic microorganisms [Citation4–12] and this property has been exploited in pharmacological applications.
West et al. in their attempts to establish structure-biological activity relationship have studied numerous transition metal complexes with heterocyclic thiosemicarbazones [Citation13]. Although there has been considerable interest in transition metal complexes derived from heterocyclic thiosemicarbazones and other related thiosemicarbazones but there has been little interest in terpenoid thiosemicarbazones [Citation14] and their metal complexes [Citation15]. Recently some Cu(II) and Ni(II) complexes of citronellal thiosemicarbazones have been reported for their property of inhibition of cell proliferation and apoptosis test on human leukemia cell line U937.
In continuation of our previous work on the synthesis, spectroscopic characterization and antimicrobial studies of binary complexes of some transition metals with semi/-thiosemicarbazone of citral and mixed ligand complexes containing amino acids or N-protected amino acids [Citation16–19], here we reported the preparation, spectral characterization and antimicrobial screening of mixed ligand complexes of Co(II) with thiosemicarbazone of citral (CDOTSC) and citronellal (DOTSC) and N- phthaloyl amino acids (N-phthaloyl glycine, N-phthaloyl alanine and N-phthaloyl valine).
Materials and methods
All the chemicals used were of AR grade. Solvents were purified by known methods [Citation20]. The triethylamine was distilled over KOH pellets. The complexes were analysed for their cobalt content employing standard literature procedure [Citation21] after destroying the organic matter first with aqua regia and then with concentrated sulphuric acid. Sulfur was estimated gravimetrically as BaSO4. Elemental analyses were carried out on a Elemental Vario EL III Carlo Erba 1108 analyzer. The IR spectra were recorded with KBr pellets in the 4000-225 cm− 1 range on Nicolet Megna 550 FT-IR spectrometer, electronic spectra were recorded on a Varian Cary 50 Bio Uv/Visible spectrometer. Molecular weights of ligands and complexes were measured by cryoscopic method using Beckmann's thermometer and magnetic moments were measured by Gouy method. Molar conductivities of 10− 4 M DMF solutions were measured on a μp based conductivity meter model 1601/E.
Synthesis of ligands
Preparation of thiosemicarbazones
The cis-3,7-dimethyl-2,6-octadienthiosemicarbazone (citral thiosemicarbazone; CDOTSC), 3,7-dimethyl-6-octenethiosemicarbazone (citronellal thiosemicarbazone; DOTSC) were synthesized as previously reported method [Citation17] by the reaction of citral or citronellal with thiosemicarbazide (1:1 molar ratio) in absolute ethanol in the presence of few drops of glacial acetic acid.
Preparation of N-phthaloyl amino acids
The N-phthaloyl amino acids (N-phthaloyl glycine, N-phthaloyl alanine, and N-phthaloyl valine) were synthesized by a published procedure [Citation22].
Synthesis of complexes
A weighed amount of corresponding thiosemicarbazone of citral or citronellal (5 mmol) was mixed with cobalt chloride (1.18 g, 5 mmol) solution in anhydrous ethanol (50 mL) followed by the addition of corresponding N-phthaloyl derivative of glycine, alanine or valine (5 mmol). After shaking the reaction mixture, triethylamine (1.4 mL, 10 mmol) was added dropwise with constant stirring. The reaction mixture was stirred with hot plate for 8 h and resulting solid was filtered off, washed with anhydrous ethanol and diethylether. Finally dried under reduced pressure. All the mixed ligand complexes were synthesized by the same method. The analytical data are given in and .
Table I. Analytical data for thiosemicarbazones, N-phthaloyl amino acids and Co(II) complexes.
Table II. Main IR spectral vibrations (cm−1) and electronic spectral bands (cm−1) for compounds.
Antimicrobial screening
(a) Evaluation of antibacterial activity by inhibition zone technique
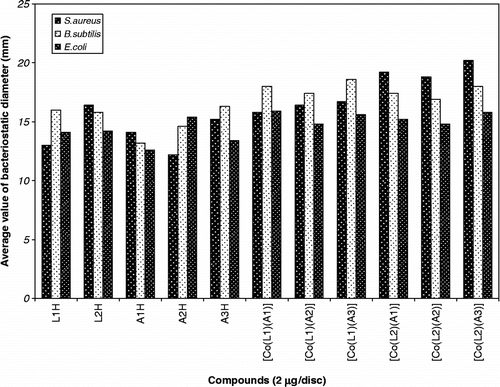
The ligands and the mixed ligand Co(II) complexes were evaluated against three bacterial strains Staphylococcus aureus, Bacillus subtilis, Gram (+ve) and Escherichia coli Gram ( − ve), using the paper disc method [Citation23]. For this purpose pure culture of bacteria were dissolved in distilled water and then uniformly seeded on the nutrient agar plate (composition: peptone 0.5%, beaf extract 3%, NaCl 5%, agar-agar 15% and distilled water 1000 mL). The paper discs of Whatman's paper No. 1 of 5 mm diameter were prepared for the purpose of making bacteriostatic slices. Ca. 2 mg of test compound was dissolved in 10 cm3 DMSO (1%) to make a concentration of 0.2 mg/cm3. The paper discs were soaked in the test solution and then such 5 paper discs were placed on Petri-dish at almost equal distance on the surface of medium preseeded with bacterial strain. One sample was inoculated in parallel on three medium plates. These Petri-dishes were incubated at 35 ± 1°C. After three days (72 h), the zone of inhibition (bacteriostatic diameter) thus formed around each disc containing the test compound was measured accurately in mm (). The relative antibacterial activity of ligands and complexes are shown in Bar graph ().
Table III. Antibacterial activity data for ligands and Co(II) complexes after 72 hours.
(b) Evaluation of antifungal activity by food poison technique
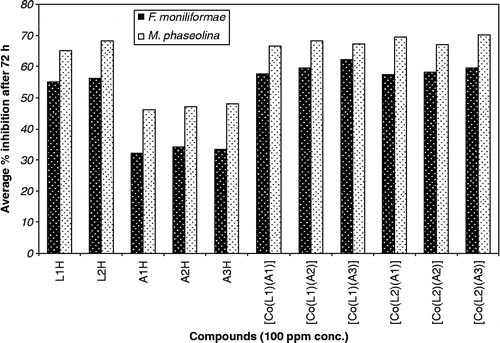
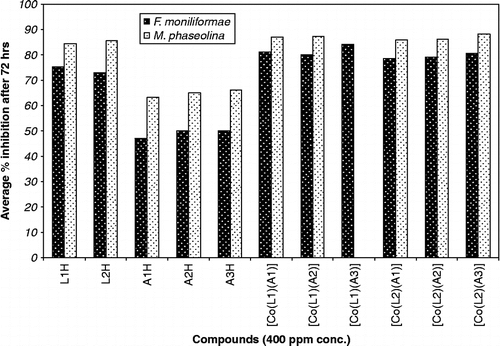
The ligands and complexes were screened for their antifungal activity against two fungal strains, Fusarium moniliformae and Macrophomina phaseolina using radial growth method [Citation24–26]. In this method the medium used was potato dextrose agar medium (composition: dextrose 15 g, agar-agar 20 g and distilled water 1000 mL). The solution of test compound was prepared by dissolving 1 mg in 1 mL of DMSO and added to known amount of medium so as to get desired concentrations (100 and 400 ppm). The flasks were shaken several times to ensure proper and uniform distribution of the test compound. The warm medium was poured in sterilized Petri-dishes and allowed to solidify. The spores of fungi were placed on the medium with the help of a sterilized inoculum needle and the Petri-dishes were placed in an incubator at 25 ± 1°C. Medium with DMSO only incubated with pathogen served as control and three replicates used in each case. The linear growth of the fungus was obtained by measuring the fungal colony after 72 h and percent inhibition was calculated ( ) according to Vincent's formula (1927) [Citation27].
Table IV. Antifungal activity data for ligands and Co(II) complexes after 72 hours.
Where C = diameter of fungal colony in control plate
T = diameter of fungal colony in test plate.
The relative antifungal activity of ligands and complexes are shown in Bar graphs ( and ).
Results and discussion
Chemistry
Reactions of cobalt(II) chloride with thiosemicarbazones (LH) and N-phthaloyl amino acids (AH) in 1:1:1 molar ratio in refluxing anhydrous ethanol in presence of triethylamine, yielded the complexes of type [Co(L)(A)](). The general reaction can be represented by Equation (1).
The analytical data of the complexes together with their molar conductances are given in the . The data are consistent with the proposed formula for the complexes. All these complexes are dark green solids, insoluble in water and in common organic solvents but soluble in DMF, DMSO and THF. The molar conductivity data () suggested the non-ionic nature of these complexes. The molecular weight measurement data indicates that these complexes are mononuclear.
IR spectra
The IR spectra of the thiosemicarbazones shows bands in the regions 3408–3475 cm− 1 and 3265–3283 cm− 1 due to the asymmetric and symmetric stretching frequencies for NH2, while the absorption for NH is present in region 3154–3162 cm− 1. An absorption band for CN appears in 1595–1622 cm− 1 regions. No band due to the SH group is observed between 2600 and 2500 cm− 1 in agreement with the thione form of thiosemicarbazone and with the presence of a band in 820–836 cm− 1 region for CS. In the IR spectra of N-phthaloyl amino acids, the carboxylic OH (except for N-phthaloyl glycine) is observed at ∼3400 cm− 1 as a broad band while a ν(OH)deformation appeared as a sharp band at ∼900 cm− 1. The band observed at ∼1750 cm− 1 may be assigned to ν(CO)asym (imido) vibration and the band observed at 1700 cm− 1 is due to the mixing of ν(CO)sym(imido) and ν(COO)asym vibrations. The ν(COO)sym band observed at ∼1400 cm− 1 is a weak band. The value of Δν = ν(COO)asym − ν(COO)sym has been found to be in the range 300–320 cm− 1 for these N-phthaloyl amino acids.
A study and comparison of IR spectra of thiosemicarbazones (CDOTSC, DOTSC), N-phthaloyl amino acids and their mixed ligand cobalt(II) complexes imply that both types of ligands behaves as monobasic bidentate ligand. The ν(C = N) absorption band of thiosemicarbazone moieties shifted towards lower wave number in the complexes, indicates coordination of the azomethine nitrogen [Citation28,Citation29] to cobalt(II) ion. This was further supported by the appearance of a new medium intensity band in the region 465–490 cm− 1, assigned to (Co–N) mode. The weak band in the region 928–1092 cm− 1 in thiosemicarbazone moieties spectra, which has ν(C = S) character, disappears in these complexes owing to the change in the nature of NH–C( = S)–NH2 on complexation [Citation30,Citation31]. A medium intensity band in the region 820–836 cm− 1 in the spectra of free thiosemicarbazones, assigned to the thioamide IV band, which has a significant contribution from ν(CS) shift to 740–759 cm− 1 in complexes, indicating coordination of sulfur atom. In addition, a band in the region 345–392 cm− 1 was assigned to ν(Co–S) and confirms coordination of the thione/thiolato sulfur atom.
The broad band appearing around ∼1700 cm− 1 due to ν(CO)sym +ν(COO)asym in the spectra of N-phthaloyl amino acids is splits into two after complexation [Citation32]. The sharp band at ∼1700 cm− 1 and a medium intensity band at 1568-1587 cm− 1 may be due to ν(CO)sym and ν(COO)asym vibrations, respectively. The magnitude of Δν [Δν = ν (COO)asym − ν(COO)sym] for these complexes found to be in the range 140–163 cm− 1, indicates chelating nature of the carboxylic group [Citation33] of N-phthaloyl amino acids and this was further supported by the appearance of a new medium intensity band in the region 422-447 cm− 1, assigned to ν(Co–O) mode.
Magnetic moments and electronic spectra
The magnetic moment measurement data () of representative complexes indicates that these cobalt(II) complexes are paramagnetic and show magnetic moment 4.12–4.56 B.M., corresponding to three unpaired electrons and indicative of tetrahedral geometry [Citation34]. The electronic spectra of these complexes exhibits two bands in the ranges 5521–5821 cm− 1 and 15225–15607 cm− 1, which may be assigned to 4A2 → 4T1 (F) and 4A2 → 4T1 (P) transitions respectively, which is characteristics of tetrahedral geometry [Citation35–37] of the complexes [].
Antimicrobial activity
The antibacterial and antifungal activity results, presented in and and respectively, show clearly that all the newly synthesized ligands and Co(II) complexes possess good antimicrobial activity. New derivatives were screened for their antibacterial activity against S. aureus, B. subtilis and E. coli and for antifungal activity against F. moniliformae and M. phaseolina which exhibited a markedly enhancement of activity on further coordination with the cobalt(II) ions against all the test bacteria / fungal strains.
The susceptibility of bacteria towards complex compounds were tested by measuring the bacteriostatic diameter (d) and compared with parent ligands with a concentration of 2.0 μg/disc, d ≥ 20 mm shows high sensitivity, 14 ≤ d < 20 mm shows medium sensitivity and 9 ≤ d ≤ 13 shows slight sensitivity. The antibacterial activity data shows that Gram + ve bacteria, S. aureus and B. subtilis are more susceptible to test compounds than the Gram − ve bacteria, E. coli, which is in accordance with previous studies. The weak antibacterial activity against Gram − ve bacteria was ascribed to the presence of an outer membrane [Citation38], which possessed hydrophilic polysaccharide chains as a barrier to hydrophobic test compound.
All the complexes show high activity against such fungi at the lower concentration and the inhibition of fungal growth has been found to be dependent on the concentration of compound. The antifungal activity data of test compounds against F. moniliformae and M. phaseolina, indicates that the test compound are more active against M. phaseolina than the F. moniliformae. It is observed from these tests that cobalt chelates have a higher activity than the free ligands. Such increased activity of the cobalt chelates can be explained on the basis of Overtone's concept [Citation39] and Tweedy's chelation theory [Citation40]. According to Overtone's concept of cell permeability, the lipid membrane that surrounds the cell favours the passage of only lipid-soluble material due to which liposolubility is an important factor that controls antibacterial activity. On chelation, the polarity of the metal ion is reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor group. Further, it increase the delocalization of π-electrons over the whole chelates ring and enhance the penetration of the complexes into lipid membranes and blocks the of metal binding sites on the enzymes of the microorganism. These complexes also disturb the respiratory processes of the cell and thus block the synthesis of protein, which restricts further growth of the organism.
Conclusion
The mixed ligand cobalt(II) complexes isolated during the present study demonstrated that the interaction of cobalt(II) chloride with thiosemicarbazone of citral or citronellal and N-phthaloyl amino acids leads to complexes with 1:1:1 stoichiometry and are found to be mononuclear. The bidentate nature of both type of ligands have been suggested on the basis of spectral evidences. All the complexes showed enhanced antimicrobial activity than the parent ligands.
Acknowledgements
The authors are greatly indebted to Prof. A. K. Bhargava and Prof. A. K. Mathur, Department of Plant Pathology, Agriculture Research Station, Durgapura, Jaipur for providing laboratory facility for carrying out antimicrobial study. The authors are also thankful to the CDRI, Lucknow for recording elemental analysis.
References
- G (a) Domgk, R Behnisch, F Mietzch, and H Smidt. (1946). Naturewissenchaften 33–315. (b) Campbell MJM. Transition metal complexes of thiosemicarbazide and thiosemicarbazones. Coord Chem Rev 1975; 15: 279
- H Stunzi. (1982). Can chelation be important in the antiviral activity of isatin β-thiosemicarbazones?. Aust J Chem 35:1145.
- VV Zyelyentsov, IG Martgnova, and NA Moskva. (1985). J Inorg Chem (Russ) 30:3130.
- ZH Chohan, H Pervez, KM Khan, and CT Supuran. (2005). Organometallic-based antibacterial and antifungal compounds: Transition metal complexes of 1,1′-diacetylferrocene-derived thiocarbohydrazone, carbohydrazone, thiosemicarbazone and semicarbazone. J Enz Inhib Med Chem 20:81.
- PK Panchal, HM Parekh, PB Pansuriya, and MN Patel. (2006). Bactericidal activity of different oxovanadium(IV) complexes with Schiff bases and application of chelation theory. J Enz Inhib Med Chem 21:203.
- M Hassan, ZH Chohan, A Scozzafava, and CT Supuran. (2004). Carbonic anhydrase inhibitors: Schiff's bases of aromatic and heterocyclic sulfonamides and their metal complexes. J Enz Inhib Med Chem 19:263.
- ZH Chohan, CT Supuran, and A Scozzafava. (2004). Metal binding and antibacterial activity of ciprofloxacin complexes. J Enz Inhib Med Chem 20:303.
- ZH Chohan, M Hassan, KM Khan, and CT Supuran. (2005). In-vitro antibacterial, antifungal and cytotoxic properties of sulfonamide derived Schiff's bases and their metal complexes. J Enz Inhib Med Chem 20:183.
- SU Rehman, ZH Chohan, F Gulnaz, and CT Supuran. (2005). In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. J Enz Inhib Med Chem 20:333.
- X Zhu, C Wang, Z Lu, and Y Dang. (1997). Synthesis, characterization and biological activity of the schiff base derived from 3,4-dihydroxybenzaldehyde and thiosemicarbazide and its complexes with nickel (II) and iron (II). Transition Met Chem 22:9.
- H Beraldo, and D Gambino. (2004). The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini Rev Med Chem 4:31.
- NC Kasuga, K Sekino, M Ishikawa, A Honda, M Yokoyama, S Nakano, N Shimada, C Koumo, and K Nomiya. (2003). Synthesis, structural characterization and antimicrobial activities of 12 zinc(II) complexes with four thiosemicarbazone and two semicarbazone ligands. J Inorg Biochem 96:298.
- DX (a) West, CS Carlson, AE Liberta, and JP Scovill. (1990). The chemical and antifungal properties of the copper(II) complexes of 2-acetylpyrazine4N-methyl-,4N-dimethyl-, and 3-hexamethyleneiminylthiosemicarbazone. Transition Met Chem 15:383. (b) West DX, Ooms CE, Saleda JS, Gebremedhin H, Liberta AE. Copper (II) and nickel(II) complexes of 2-formylpyridine 3-piperidinyl-, 3-hexamethyleneiminyl- and 3-azabicyclo[3.2.2]nonylthiosemicarbazones. Transition Met Chem 1994;19:553
- P Tarasconi, S Capacchi, G Pelosi, M Cornia, R Albertini, A Bonati, PP Dall'Aglio, P Lunghi, and S Pinelli. (2000). Synthesis, spectroscopic characteization and biological properties of new natural aldehydes thiosemicarbazones. Bioorg Med Chem 8:157.
- MB Ferrari, F Bisceglie, G Pelosi, M Sassi, P Tarasconi, M Cornia, S Capacchi, R Albertini, and S Pinelli. (2002). Synthesis, characterization and X-ray structures of new antiproliferative and proapoptotic natural aldehyde thiosemicarbazones and their nickel(II) and copper(II) complexes. J Inorg Biochem 90:113.
- R Sharma, SK Agarwal, S Rawat, and M Nagar. (2006). Synthesis, characterization and antibacterial activity of some transition metal cis-3,7-dimethyl-2,6-octadiensemicarbazone complexes in place of Synthesis, formation constant and biocidal activity of some bivalent transition metal complexes derived from cis-3,7-dimethyl-2,6-octadienphenylhydrazone. Transition Met Chem 31:201.
- R Sharma, AK Bansal, and M Nagar. (2005). Transition metal complexes of cis–3,7–dimethyl–2,6-octadienthiosemicarbazone: Synthesis, characterization and biological studies. Ind J Chem 44A:2255.
- R Sharma, and M Nagar. (2006). Synthesis, structural and antibacterial studies of some mixed ligand complexes of Zn (II), Cd (II) and Hg (II) derived from citral thiosemicarbazone and N-phthaloyl amino acids. Phosphorus, Sulfur Silicon Relat Elem 181:2863.
- M Nagar, S Rawat, R Sharma, H Sharma, and M Agarwal. (2007). Synthesis, characterization and antifungal activity of mixed ligand complexes of CoII, NiII, and CuII with cis-3,7-dimethyl-2,6-octadiensemicarbazone and amino acids. Ind Chem Soc 84:341.
- AI Vogel. A text book of practical organic chemistry. 5th ed. London: ELBS, Longman; (1985).
- AI Vogel. A text book of quantitative inorganic analysis. 5th ed. London: ELBS, Longman; (1989).
- JC Sheehan, DW Chapman, and RW Roth. (1952). The synthesis of stereochemically pure peptide derivatives by phthaloyl method. J Am Chem Soc 74:3822. Zeng Q, Liu Z, Li B, Wang F. Mild and effective N-phthaloylation of amino acids. Amino Acids 2004;27:183
- MC Bryant. Antibiotic and their laboratory control. London: Butterworth; (1988). p26.
- D Gottlieb, HH Hassan, and MB Linn. (1952). Phytopathology 218.
- YL Nene, and PN Thapliyal. (1965). Naturewissenchaften 89.
- YL Nene, and K Kumar. (1966). Naturewissenchaften 53:363.
- IM Vincent. (1927). Nature 59.
- BV Agarwala, S Hingorani, V Puri, CL Khetrapal, and GA Naganagowda. (1994). Physicochemical studies of (o-vanillin thiosemicarbazonato)-nickel(ii) chelate. Transition Met Chem 19:25.
- Y Harek, L Larabi, L Boukli, F Kadri, N Benali-Cherif, and MM Mostafa. (2005). Synthesis, characterization, crystal structure and electrochemical behavior of a nickel(II) complex of 5,6-dihydro-2H-pyran-3-aldehyde thiosemicarbazone. Transition Met Chem 30:121.
- MA Ali, and R Bose. (1977). Metal complexes of schiff bases formed by condensation of 2-methoxybenzaldehyde and 2-hydroxybenzaldehyde with S-benzyldithiocarbazate. J Inorg Nucl Chem 39:265.
- M Hurehu, and M Dima. (1968). Rev Roum Chem 13:359.
- AK Saxena, S Saxena, and AK Rai. (1990). Some titanium (IV) complexes of 1,3-dihydro-1,3-dioxo- α- (substituted)-2H-isoindole-2-acetates. Synth React Inorg Met-Org Chem 20:21.
- LH Abdel-Rahman. (2001). N-phthaloylglycinate ternary complexes with cobalt(II), nickel(II), copper(II), zinc(II), and cadmium(II), metal ions and aromatic mono, bi and tridentate amines. Transition Met Chem 26:412.
- E Canpolat, and M Kaya. (2005). Studies on mononuclear chelates derived from substituted schiff base ligands (Part 4): Synthesis and characterization of new 5-hydroxysalicyliden-P-aminoacetophenoneoxime and its complexes with Co(Ii), Ni(II), Cu(II), and Zn(II). Turk J Chem 29:409.
- J Lewis, and RG Wilkins. Modern coordination chemistry. New York: Interscience; (1967).
- L Casella, and M Gullotti. (1981). Coordination modes of histidine. 2. Stereochemistry of the reaction between histidine derivatives and pyridoxal analogs conformational properties of zinc(II) complexes of histidine Schiff bases. J Am Chem Soc 103:6338.
- ABP Lever. Inorganic electronic spectroscopy. 2nd ed. Amsterdam: Elsevier; (1984).
- CM Mann, SD Cox, and JL Markham. (2000). Lett App Microbiol 30:294.
- Y Anjaneyula, and RP Rao. (1986). Preparation, characterization and antimicrobial activity study on some ternary complexes of Cu(II) with acetyl acetone and various salicylic acids. Synth React Inorg Met-Org Chem 16:257.
- BG Tweedy. (1964). Phytopathology 55:910.
![Figure 4. The proposed structure formula for the complexes [Co(L)(A)].](/cms/asset/e4cf491e-0fc1-48bc-b30c-6fbfc92777ee/ienz_a_305294_f0004_b.gif)