Abstract
The present work focused on the kinetics of the inhibitory effects of the leaf extract of Siberian Crabapple, named Shan jingzi in China, on chicken liver fatty acid synthase. The results showed that this extract had much stronger inhibitory ability on fatty acid synthase than that from green teas described in many previous reports. The inhibitory ability of this extract is closely related to the extracting solvent, and the time of extraction was also an important influencing factor. The inhibitory types of this extract on diffeerent substrates of chicken liver fatty acid synthase, acetyl-CoA, malonyl-CoA and NADPH, were found to be noncompetitive, uncompetitive and mixed, respectively. The studies here shed a new light on the exploration for inhibitors of fatty acid synthase.
| Abbreviations: | ||
| FAS, | = | fatty acid synthase (EC 2.3.1.85) |
| NADPH, | = | reduced nicotinamide adenine dinucleotide phosophate |
| EGCG, | = | epigallocatechin gallate |
| ECG, | = | epicatechin gallate |
| EDTA, | = | ethylene diamine tetraacetic acid |
| IC50, | = | the concentration of inhibitor for half inhibition of the total enzyme activity |
Introduction
Obesity is a world wide problem which is affecting many people nowadays. Although it has been a health issue in many developed countries during the past century, its prevalence recently has increased to the extent that the World Health Organization has declared it an epidemic representing a worldwide threat to public health [Citation1]. Obesity is able to bring the sufferers many diseases, such as cardiovascular disease [Citation2], type II diabetes [Citation3], asthma and atopic diseases [Citation4], and several common adult cancers [Citation5]. As a matter of fact, it is necessary to find some medical treatments of obesity.
Fatty acid synthase (EC 2.3.1.85, FAS) catalyzes the de novo synthesis of fatty acids from acetyl- coenzyme A (acetyl - Co A) and malonyl- coenzyme A (malonyl - Co A) in the presence of the reducing substrate, reduced nicotinamide adenine dinucleotide phosophate (NADPH) [Citation6]. It was reported that FAS may play a role in the control of feeding behavior, and the treatment of mice with the synthetic FAS inhibitor C75 can result in reduced the appetite and dramatic weight loss of the mice. The accumulation of the substrate malonyl-CoA through the inhibition of FAS appears to inhibit the expression of neuropeptide Y that promotes ingestion [Citation7]. Therefore, FAS is a potential therapeutic target for obesity.
To date, many chemical-synthetic drugs have been researched and exposed activity in treating with obesity. However, most of them have some toxicity and other side effects. In recent years, more and more native-sourced plants including many herbs were found obesity-inhibiting activity and used to prevent or treat obesity.
Tea (Camellia sinensis) is one of the most familiar beverages all over the world. It has been reported that green tea and some catechin such as epigallocatechin gallate (EGCG) and epicatechin gallate (ECG) from green tea were inhibitors of FAS [Citation8].
Siberian Crabapple (Malus baccata (Linn.) Borkh, common named Shan jingzi), is a plant which distributes in many areas of China, and its fruits are sweet and can be eaten. The extracts of its leaves by boiling water are considered as weight reducing tea in many regions of China. In our work, we use this plant leaves as experiment materials to detect the weight-reducing effects of its extracts by inhibiting the FAS activity. Our research work will play an important function in finding a new obesity-cure drug.
Materials and methods
Materials
The preparation, storage and use of chicken liver FAS was performed as previously described [Citation9]. The final purified enzyme was homogeneous on polyacrylamide gel electrophoresis (PAGE) in the presence and absence of sodium dodecyl sulfate (SDS). The chicken liver FAS has a dimer molecular weight of 500,000 Da and a subunit molecular weight of 250,000 Da. Acetyl-CoA, malonyl-CoA, NADPH, ethylene diamine tetraacetic acid (EDTA) and theaflavins (purity > 80%) were purchased from Sigma. The leaves of Siberian Crabapple were purchased from Shan Dong province of China. All other reagents were local products of analytical grade.
The enzyme and reagents concentrations were determined by absorption measurements using the extinction coefficients according to the method described previously [Citation9].
The leaves of Siberian Crabapple were extracted as following. Crushed leaves (1.0 g) were put into 20 mL of respective solvent and stirred for 2.0 h at room temperature to give the extract.
Assays
Activities of FAS for the overall reaction, ketoacyl reduction and enoyl reduction were determined with an Amersham Pharmacia Ultrospec 4300 pro UV–Vis Spectrometer at 37°C by following the decrease in NADPH at 340 nm. The assay solution used for these reactions has been described previously [Citation10]. The reaction mixture for the overall reaction, (M1), contained 100 mmol/L potassium phosphate buffer, pH 7.0, 1 mmol/L EDTA, 3 mmol/L acetyl-CoA, 10 mmol/L malonyl-CoA, 35 mmol/L NADPH and 10 μg FAS in a total volume of 2.0 mL. The ketoacyl reduction assay mixture, (M2), contained 40 mmol/L ethyl acetoacetate, 35 mmol/L NADPH, 1 mmol/L EDTA and 10 μg FAS in 100 mmol/L potassium phosphate buffer, pH 7.0. The enoyl reduction reaction mixture, (M3), contained 40 mmol/L ethyl crotonate, 35 mmol/L NADPH, 1 mmol/L EDTA and 60 μg FAS in 10 mmol/L potassium phosphate buffer, pH 6.3.
The inhibitor solution (1–10 μL) was added to the assay mixture, followed by the FAS solution to start the reaction in a total volume of 2.0 mL. The activity of FAS was assayed as Ai, and the control activity with the same solvent only was assayed as A0, and Ai/A0 was taken as the relative activity. This inhibition was caused by the non-covalent fast combination of inhibitor with enzyme and was usually reversible. The concentration of inhibitor for half inhibition of the enzyme activity (IC50) was obtained from a plot of residual activity versus inhibitor concentration.
The residual enzyme activity was detected by adding 1 μL extract solution at different extraction times and the inhibition time course was the relative activity of FAS to the native state versus time. The extracting time was 160 min. From this experiment, we can determine the optimum extracting time in the respective extracting solvent.
Kinetics
The inhibition kinetics of the extract to FAS was studied in the assay mixture containing the inhibitor extracted by 50% ethanol. After the enzyme solution was mixed with inhibitor solution (in 50% ethanol) with the ratio of 50:1 (v/v), aliquots were taken to measure activity at the indicated time intervals. It was checked that 1% ethanol did not cause any changes in FAS activity at the indicated time intervals (data not shown). The activity of native enzyme was taken as 100%. The time course of remaining activity was obtained because of its characteristic time-dependence and this inhibition was chiefly irreversible due to the covalent reaction of the inhibitor with the enzyme. The rate constant kobs of inactivation was obtained from the slope of a semilog plot of the time course.
Results
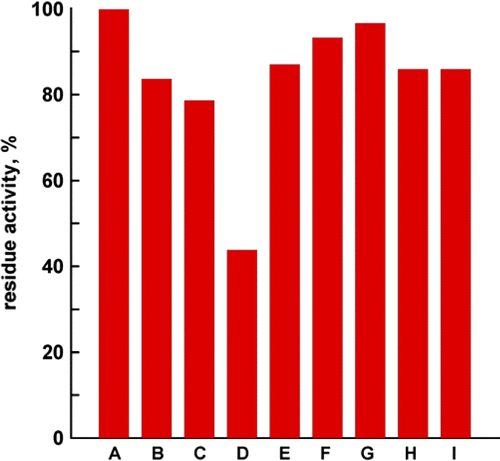
Different solvents with various polarities were examined to extract the effective components with inhibition activity on FAS. The results in showed that the residual activity of FAS (shown as the height of each column) in the assay system with the extracts by 50% ethanol solution was the lowest among all extraction solvents, which was only about 40% activity of the native enzyme. The FAS inhibiting ability of each extract with different solvents decreased in the following order: 50%ethanol > 25%ethanol > water > light petroleum > n-butanol > 75%ethanol > 100%ethanol, and acetone. Except for 50% ethanol, the residual activity of FAS in the assay system with other solvents was not less than 75% of the native enzyme. That means that most of the FAS inhibitors were extracted in 50% ethanol solution. Consequently, we considered 50% ethanol solution as the optimal solvent for extracting the FAS inhibitor from Siberian Crabapple leaves.
Figure 1. Extraction efficiency by different solvents. The dry leaves of Siberian Crabapple (1 g) were dipped into 20 mL different solvents, stirred for 2 h, and then the extracts were collected. 1 μL of each extract solution was added to the assay system to determine the overall reaction activity, abbreviated as OA. The reaction was initiated by adding FAS, with a final enzyme concentration of 0.02 mmol/L. a, 1 μL potassium phosphate buffer was added into the assay system, and the activity of FAS under this condition was normalized as 100%; the solvents of extracts adding to assay system from b to i were water, 25%ethanol, 50%ethanol, 75%ethanol, 100%ethanol, acetone, n-butanol, light petroleum, respectively.
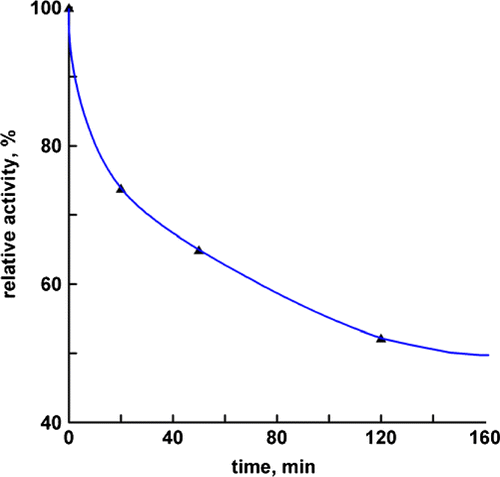
shows the inhibitory effects of the extracts in 50% ethanol at different extraction times on FAS. Residual activity of FAS initially decreased quickly in the early stages of the extraction but after 120 min, the change in the enzyme activity was not obvious. As indicated in the Figure, the extracts with longer extraction time showed increased inhibitory effects on FAS. Most of the FAS inhibitors were extracted in 120 min by 50% ethanol. So we extracted the FAS inhibitors in 120 min in the followed experiments, except for the inhibition kinetics.
Figure 2. The inhibition activity of extract according to extracting time. To determine the appropriate extracting time for effective factors in inhibiting the FAS activity. The FAS solution and extract (in 50% ethanol) were mixed in the ratio 50:1 (v/v) in 0.1 mol/L phosphate buffer, pH 7.0, the final concentrations of FAS and extract being 1.9 mmol/L and 100.6 μg dry weight/mL respectively, and aliquots were taken immediately to the incubated assay mixture and detected the remaining activity of FAS at the indicated time intervals.
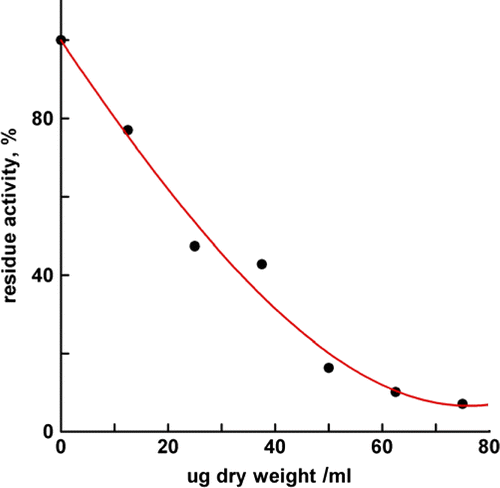
IC50, half-inhibition concentration, means the dry weight of extract per milliliter extract solvent to inhibit half of the enzyme activity of FAS. The IC50 value of the extract with 50% ethanol is shown in . From these data, it can be seen that the IC50 of this extract is 27.1 μg dry weight/mL. This extract exhibited much stronger inhibitory ability on FAS than several well known Chinese teas, for example, the IC50 of Xihulongjing Green Tea and Oolong Tea to FAS were approximately 40 and 60 μg dry weight/mL, respectively [Citation11].
Figure 3. Concentration effect of the extract on the inhibition on FAS. The extract was in 50% ethanol. The final enzyme concentration was 0.02 mmol/L.
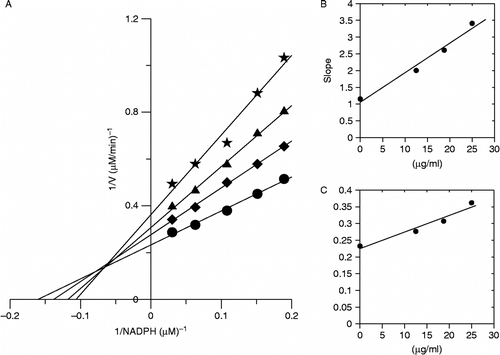
The reversible inhibition kinetics of the 50% ethanol extract on the overall activity of FAS for all the three substrates were studied. The results are shown in , and . In , Kis = 19.5 μg/mL, Kii = 26.6 μg/mL. From the double-reciprocal plots it could be estimated as noncompetitive inhibition between the extract and Ac-CoA to FAS activity In , Kii = 18.91 μg/mL. From the double-reciprocal plots it could be estimated as uncompetitive inhibition between the extract and malonyl CoA to FAS activity. In , Kis = 11.9 μg/mL, Kii = 45.36 μg/mL. From the double-reciprocal plots it could be estimated as mixed inhibition between the extract and NADPH to FAS activity.
Figure 4. Inhibitory kinetics of the extract on the activity of FAS against acetyl-CoA. Final concentrations of the inhibitor in the systems were: (•) 0 μg/mL; (♦) 9.375 μg/mL; (▴) 18.75 μg/mL; (*) 28.125 μg/mL. Different concentrations of Acetyl-CoA were 0.5, 0.7, 0.9, 1.1 and 1.3 μmol/L, respectively. The final concentrations of malonyl-CoA and NADPH in the reaction system were 10 μmol/L and 35 μmol/L, respectively. The slopes (B) and intercepts (C) of the curves in Figure 4(A) were plotted against different concentrations of the inhibitors.
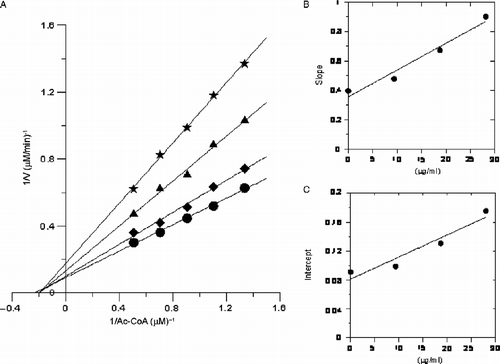
Figure 5. Inhibitory kinetics of the extract on the activity of FAS against malonyl-CoA. The final concentration of the inhibitor in the systems were: (•) 0 μg/mL; (♦) 12.5 μg/mL; (▴) 18.75 μg/mL; (*) 25 μg/mL. Different concentrations of malonyl-CoA were 0.2, 0.3, 0.4 and 0.5 μmol/L, respectively. The final concentrations of acetyl-CoA and NADPH were 5 μmol/L and 5 μmol/L, respectively.
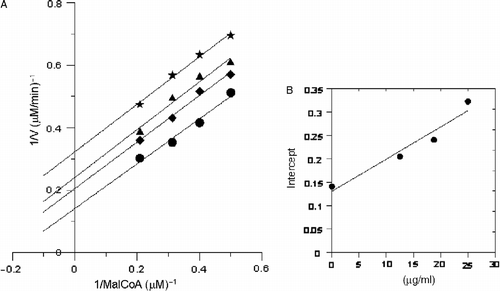
Figure 6. Inhibitory kinetics of the extract on the activity of FAS against NADPH. The final concentrations of the inhibitor in the systems were: (•) 0 μg/mL; (♦) 12.5 μg/mL; (▴) 18.75 μg/mL; (*) 25 μg/mL. Different concentrations of NADPH were 0.03, 0.06, 0.1, 0.15 and 0.18 μmol/L, respectively. The final concentrations of acetyl-CoA and malonyl-CoA were 5 μmol/L and 10 μmol/L, respectively.
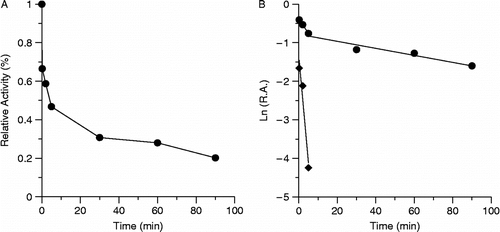
The time course of the inhibition of the extract on FAS activity is shown in . From Figure (B), the whole inhibition process of the extract to FAS could be divided into a rapid phase and a slow phase, and the observed equal constants were Kobs = 0.556 min− 1 according to rapid phase and Kobs = 0.0091 min− 1 according to slow phase, respectively. It is indicated that the inhibition of the extract on FAS was time-dependent, which infers that there was irreversible inhibition on FAS by this extract during the whole inhibition course.
Figure 7. Time course effect of the extract on the inhibition of FAS. The final concentrations of FAS and extract being 1.9 mmol/L and 100.6 μg dry weight/mL respectively. Figure (A) was the changes of residual activity of FAS. Figure (B) was the semi-logarithm curve of Figure (A), and R.A. means the relative activity to the control. The two lines means the whole inhibition process can be divided into two stages, which have different inhibition rates, which exhibited as different slopes of the two lines.
Discussion
Obesity is considered as the maladjustment of two physiological functions, energy metabolism and appetite regulation Citation12–14. The central nervous system (CNS) regulates the feeding behavior according to its evaluation, integration and regulation of energy status of the whole body Citation15–18. Recently, more and more research results exposed that the potential role of fatty acid biosynthesis and metabolism could not be ignored in this process. And the regulation of the fatty acid biosynthesis and metabolism can control the energy state and food intake distinctly [Citation12,Citation19,Citation20]. FAS is a key enzyme within the fatty acid metabolism pathway, catalyzes the de novo synthesis of fatty acids from acetyl-coenzyme A (acetyl - Co A) and malonyl- coenzyme A (malonyl - Co A). So it becomes an important regulation point in the regulation and control of fatty acid metabolism and even the appotite and weight.
In recent years, the extracts of many plants, including some green tea, black tea and many other herbs, have been reported to contain inhibitors of FAS, and most of them could reduce body weight Citation11,Citation19–22. Our work indicated that the ethanol extracts from the leaves of Siberian Crabapple also contained effective inhibition capability on FAS activity, and its inhibitory potency was higher than any other tea extract.
The extraction method remarkably affects the efficiency of extracting FAS inhibitors. Among the solvents used in this experiment, 50% ethanol was the optimal one. It was reported that only 10–23% of the inhibitory activity from the black tea was extracted by the general method of boiling water, and the extracting efficiency of 50% ethanol is over 4-fold more than boiling water [Citation11]. In our work, only about 15% activity of FAS was inhibited by the water extraction extract at room temperature, whereas, more than 50% of FAS was inhibited by the 50% ethanol extract. Both the above results indicated that ethanol could extract the FAS inhibitors more effectively than the generally used water. At the same time, it was found that the temperature of the extracting solvents did not markedly affect efficiency of extracting the FAS inhibitors.
The inhibition kinetics results showed that the inhibition of the 50% ethonal extract to FAS was a complicated process, which included a fast reversible inhibition process and a slow irreversible inhibition process. The inhibition of FAS displayed different inhibition types towards different substrates of FAS, for instance, it exhibited noncompetitive inhibition to Acyl-CoA, uncompetitive inhibition to malonyl-CoA, and mixed inhibition to NADPH.
According to our results, this extract from a natively-sourced plant can inhibit the activity of FAS effectively in vitro. Further research work on it, such as determination of the structural components, the inhibition effects to FAS in vivo, could probably make it a promising fat-reduction drug in future.
Acknowledgements
This work was supported by the National Natural Science Foundations of China (30670460, 30770475), the Science and Technology Planning Project of Zhejiang Province of China (2007C24021), the Science and Technology Planning projects of Jiaxing, Zhejiang, China (2007AZ1015, 2007A36) and the Qianjiang Program of Zhejiang Province, China (2006R10036).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- A Lang, and ES Froelicher. (2006). Management of overweight and obesity in adults: Behavioral intervention for long-term weight loss and maintenance. Eur J Cardio Nur 5 (2):102–114.
- DL McGee. (2005). Body mass index and mortality: A meta-analysis based on person-level data from twenty-six observational studies. Ann Epi 15 (2):87–97.
- KS Calderon, CB Yucha, and SD Schaffer. (2005). Obesity-related cardiovascular risk factors: Intervention recommendations to decrease adolescent obesity. J Ped Nur 20 (1):3–14.
- M Kilpeläinen, M Koskenvuo, H Helenius, and et al (2001). Wood stove heating, asthma and allergies. Resp Med 95 (11):911–916.
- AG Renehan, J Frystyk, and A Flyvbjerg. (2006). Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endo & Met 17 (8):328–336.
- S Smith. (1952). Study in the development of the rainbow trout (Salmo Irideus): II. The metabolism of carbohydrates and fats. J Exp Biol 29:650–666.
- TM Loftus, DE Jaworsky, GL Frehywot, and et al (2000). Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288:2379–2381.
- WX Tian, LC Li, XD Wu, and et al (2004). Weight reduction by Chinese medicinal herbs may be related to inhibition of fatty acid synthase. Life Sci 74 (19):2389–2399.
- WX Tian, RY Hsu, and YS Wang. (1985). Studies on the reactivity of the essential sulfhydryl groups as a conformational probe for the fatty acid synthetase of chicken liver. Inactivation by 5,5′-dithiobis-(2-nitrobenzoic acid) and intersubunit cross-linking of the inactivated enzyme. J Biol Chem 260 (20):11375–11387.
- X Wang, and WX Tian. (2001). Green tea epigallocatechin gallate: A natural inhibitor of fatty-acid synthase. Biochem Biophys Res Commun 288 (5):1200–1206.
- YT Du, X Wang, XD Wu, and et al (2005). Keemun black tea extract contains potent fatty acid synthase inhibitors and reduces food intake and body weight of rats via oral administration. J Enz Inhib Med Chem 20 (4):349–356.
- GV Ronnett, EK Kim, LE Landree, and et al (2005). Fatty acid metabolism as a target for obesity treatment. Physiol Behav 85:25–35.
- SM Gale, VD Castracane, and CS Mantzoros. (2004). Energy homeostasis,obesity and eating disorders: Recent advances in endocrinology. J Nutr 134:295–298.
- PM Hellstrom, A Geliebter, E Naslund, PT Schmidt, EK Yahav, SA Hashim, and et al (2004). Peripheral and central signals in the control ofeating in normal, obese and binge-eating human subjects. Br J Nutr 92 (Suppl. 1):S47–S57.
- S Woods, R Seeley, DJ Porte, and M Schwartz. (1998). Signals that regulate foodintake and energy homeostasis. Science 280:1378–1383.
- PJ Magistretti. Brain energy metabolism Fundamental neuroscience. Academic Press. (2004); p 67–89.
- M Schwartz, S Woods, DJ Porte, R Seeley, and D Baskin. (2000). Centralnervous system control of food intake. Nature 404:661–671.
- BM Spiegelman, and JS Flier. (2001). Obesity and the regulation of energybalance. Cell 104:531–543.
- WX Tian, LC Li, XD Wu, and et al (2004). Weight reduction by Chinese medicinal herbs may be related to inhibition of fatty acid synthase. Life Sci 74:2389–2399.
- MK Na, JP Jang, BS Min, and et al (2006). Fatty acid synthase inhibitory activity of acylphloroglucinols isolated from Dryopteris crassirhizoma. Bioorg Med Chem Let 16:4738–4742.
- YH Kao, RA Hiipakka, and S Liao. (2000). Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 141 (3):980–987.
- AG Dulloo, J Seydoux, L Girardier, and et al (2000). Green tea and thermogenesis: Interactions between catechin-polyphenols, caffeine and sympathetic activity. Inte J Obes Rel Met Dis 24 (2):252–258.