Abstract
The whole plants of Carpesium genus are used in traditional medicine as anti-pyretic, analgesic and vermifugic, including a topical application for sores and inflammation. A previous study on Carpesium genus suggested that the antiplasmodial activity against Plasmodium falciparum was due to the existence of 11(13)- dehydroivaxillin (DDV) from EtOAc extracts of C. ceruum (Compositae). Here, the antimalarial activity of DDV was evaluated against Plasmodium berghei in mice. The LD50 of the compound was determined as 51.2 mg/kg, while doses of 124 mg/kg and above were found to be lethal to mice. DDV (2, 5, 10 mg/kg/day) exhibited a significant blood schizontocidal activity in 4-day early infection, repository evaluation and in an established infection with a significant mean survival time comparable to that of the standard drug, chloroquine, 5 mg/kg/day. DDV possesses a promising antiplasmodial activity, which can be exploited in malaria therapy.
Introduction
Malaria is the major tropical disease due to parasites, responsible for significant morbidity and mortality in the world [Citation1]. A dramatic recrudescence of malaria is ongoing due to the increasing resistance of vectors to insecticides and the progressive resistance of the parasite, mainly Plasmodium falciparum, to drugs. These developments and the difficulty of creating efficient vaccines underline the urgent need for new antimalarial drugs [Citation2]. The situation is aggravated by the occurrence of parasites resistant to both chloroquine and alternative drugs such as mefloquine, pyrimethamine/sulfadoxine and even quinine [Citation3]. We began to investigate experimentally with in vitro malaria models the antimalarial activity of the plants used experientially as the ingredients of various prescriptions in the traditional medical plants in South Korea. Our previous study revealed that the EtOAc extracts from dried whole parts of C. ceruum had a high in vitro antimalarial activity against Plas. falciparum. We have reported isolation, structure elucidation and in-vitro antimalarial activity of a sesquiterpene lactone (11(13)-dehydroivaxillin; DDV) from C. ceruum [Citation4]. In the present study, we demonstrate that the DDV isolated from the whole parts of C. ceraum shows antimalarial activity on Plas. berghei in mice. The DDV showed potential in vivo activity against Plas. berghei in our screening program.
Material and methods
Isolation of 11(13)-dehydroivaxillin (DDV) and animals used
Isolation of DDV was reported previously in detail [Citation4]. Outbred male ICR mice, 8 weeks old, purchased from Japan SLC Inc., were used as a host. The animals were housed in standard cages and acclimatized for a period of 10 days. The mice were maintained on standard feed and water ad libitum. All animal experiments were performed according to the guidelines for animal experimentation, Sanbon Medical Center, Wonkwang University. Rodent malaria parasite, Plas. berghei, was maintained by serial blood passage in mice. Blood stage parasites were stored at − 80°C.
Parasite inoculation
The chloroquine sensitive Plas. berghei (ATCC 50175) was obtained from American Type Culture Collection, Manassas, VA, USA and maintained in mice. The inoculum consisted of 5 × 107 Plas. berghei parasitized red blood cells per mL. This was prepared by determining both the percentage parasitaemia and the red blood cell count of the donor mouse and diluting the blood with isotonic saline in proportions indicated by both determinations. Each mouse was inoculated intraperitoneally on day 0, with 0.2 mL of infected blood containing about 1 × 107 Plas. berghei parasitized red blood cell. The LD50 of the compound was determined using male ICR mice by the intraperitoneal route using the method of Lorke et al. [Citation5].
Evaluation of schizontocidal activity on early infection (4-day test)
A method described by Knight and Peters was used [Citation6]. The animals were divided into five groups of five mice each and were orally administered with 2, 5 and 10 mg/kg/day of test compound, chloroquine 5 mg/kg/day (positive control) and an equivalent volume of distilled water (negative control group) for 4 consecutive days (day 0 to day 3) between 8.00 a.m. and 9.00 a.m. On the fifth day (D 4), 24 h after the administration of the last dose, thin blood films were made from the tail blood and stained with Giemsa stain and the percentage parasitaemia was determined by counting the number of parasitized erythrocytes out of 200 erythrocytes in random fields of the microscope. Average percentage chemosuppression was calculated as 100[(A − B)/A], where A is the average percentage parasitaemia in the negative control group and B is the average parasitaemia in the test group.
Evaluation of the repository activity
The repository activity was assessed by using the method described by Peters et al. [Citation7]. The mice were divided into five groups of five mice each and administered orally with 2, 5 and 10 mg/kg doses of the test compound, 1.2 mg/kg/day pyrimethamine (positive control) and distilledwater (negative control) for 4 consecutive days (D0–D3). On day 5 (D4), the mice were inoculated with Plas. berghei. Seventy-two hours later, the parasitaemia level was assessed by blood smears.
Evaluation of schizontocidal activity in established infection (Rane test)
A modified method similar to that described by Ryley and Peters was used [Citation8]. On the first day (day 0), standard inoculum of 1 × 107 Plas. berghei infected erythrocytes was injected intraperitoneally into mice. Seventy-two hours later, the mice were divided into five groups of five mice each. Different doses of compound (2, 5 and 10 mg/kg/day) were administered orally to these groups. Chloroquine (5 mg/kg/day) was given to the positive control group and an equal volume of distilled water to the negative control group. The drug/compound was given once daily for 5 days. Thin films stained with Giemsa stain were prepared from tail blood of each mouse daily for 5 days to monitor the parasitaemia level. The mean survival time for each group was determined arithmetically by finding the average survival time (days) of the mice (post-inoculation) in each group over a period of 30 days (D0–D29). The parasitaemia level of the animals that survived after 30 days of monitoring were determined using thin blood film made from tail blood of each surviving animal.
Statistical analysis
Data obtained from the study were analyzed statistically using Student's-test and values of P < 0.05 were considered significant.
Result and discussion
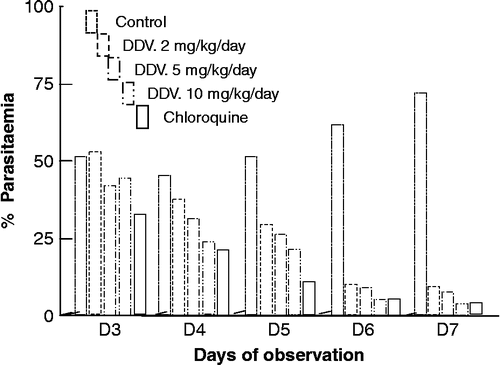
DDV (5–200 mg/kg; ) produced signs of toxicity on mice treated with it depending on the dose administered ranging from writhing, gasping, decreased respiratory rate, decreased limb tone and death. In this study, the acute toxicity evaluation of the compound revealed that doses of 100 mg/kg and above were lethal to the animals and the determined LD50 of the compound, 51.4 mg/kg shows that the extract is moderately toxic. Animals treated with 124 mg/kg and above of the compound died. DDV isolated from C. ceruum produced a dose-dependent chemosuppression effect at the different doses of the compound employed (2, 5, and 10 mg/kg) and administered orally causing significant chemosuppressions () which were significant when compared to control. The standard drug, chloroquine, caused a significant higher chemosuppression than that of the compound-treated group (). DDV exerted a dose-dependent repository activity at the various doses employed (2, 5, and 10 mg/kg/day) causing significant chemosuppression (). There was a dose-dependent reduction in parasitaemia of the compound-treated group, while the control group showed a daily increase in parasitaemia. Chloroquine (5 mg/kg/day) also produced a daily reduction in parasitaemia. The percentage parasitaemia of the compound treated groups on day 7 as well as that of control and chloroquine treated groups are shown in . The mean survival time (m.s.t.) of the mice in various groups were 23.0, 28.0, 30.0, 30.0 and 16 days for 2, 5 and 10 mg/kg/day of compound, chloroquine and control groups, respectively. The animals that survived in the compound-treated group as well as chloroquine group were found to be parasite free. The results indicate that DDV possesses blood schizontocidal activity as evident from the chemosuppression obtained during the 4- day early infection test. The compound also exhibited repository activity, though the doses employed could not produce suppression comparable to that of the standard drug (pyrimethamine 1.2 mg/kg). A significant activity was also recorded during an established infection, which was comparable to the standard drug (chloroquine, 5 mg/kg/day). The highest dose of the compound (10 mg/kg/day) was observed to sustain the mice throughout the 30-day period of study similar to that of the standard drug, chloroquine. Although the mechanism of action of this compund has not been elucidated, some plants and/or compounds are known to exert antiplasmodial action either by causing elevation of red blood cell oxidation [Citation9] or by inhibiting protein synthesis [Citation10]. The compound could have elicited its action through either of the two mechanism mentioned above or by some other unknown mechanism.
Table I. Blood schizontocidal activity of 11(13)-dehydroivaxillin (DDV) treated Plasmodium berghei infection in mice.
Acknowledgement
Jong-Jin Kim and Ill-Min Chung equally contributed to this work.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- W Trager, and JB Jensen. (1976). Science 193:673–675.
- C Clarkson, VJ Maharaj, and NR Crouch. (2004). J Ethnopharmacol 92:177–191.
- A Biorjman. Malaria. Waiting for the vaccine. GAT target. Chichester: John Wiley and Sons; (1991).
- IM Chung, and HI Moon. (2007). J Enz Inhib Med Chem JEIMC-07/1363:In press.
- D Lorke. (1983). Arch Toxicol 54:275–287.
- DJ Knight, and W Peters. (1980). Ann Trop Med Parasitol 74:393–404.
- W Peters. (1965). Exp Parasitol 17:80–89.
- JF Ryley, and W Peters. (1970). Ann Trop Med Parasitol 84:209–222.
- NL Etkin. (1997). Trop Doct 27:12–16.
- GC Kirby, MJ O'Neil, JD Philipson, and DC Warhurst. (1989). Biochem Pharmacol 38:4367–4374.