Abstract
Silymarin, a known standardized extract obtained from seeds of Silybum marianum is widely used in treatment of several diseases of varying origin. In the present paper, we clarified the antioxidant activity of silymarin by employing various in vitro antioxidant assay such as 1,1-diphenyl-2-picryl-hydrazyl free radical (DPPH·) scavenging, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging activity, total antioxidant activity determination by ferric thiocyanate, total reducing ability determination by Fe3+ − Fe2+ transformation method and Cuprac assay, superoxide anion radical scavenging by riboflavin/methionine/illuminate system, hydrogen peroxide scavenging and ferrous ions (Fe2+) chelating activities. Silymarin inhibited 82.7% lipid peroxidation of linoleic acid emulsion at 30 μg/mL concentration; butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), α-tocopherol and trolox indicated inhibition of 83.3, 82.1, 68.1 and 81.3% on peroxidation of linoleic acid emulsion at the same concentration, respectively. In addition, silymarin had an effective DPPH· scavenging, ABTS√+ scavenging, superoxide anion radical scavenging, hydrogen peroxide scavenging, ferric ions (Fe3+) reducing power by Fe3+ − Fe2+ transformation, cupric ions (Cu2+) reducing ability by Cuprac method, and ferrous ions (Fe2+) chelating activities. Also, BHA, BHT, α-tocopherol and trolox, were used as the reference antioxidant and radical scavenger compounds. Moreover, this study, which clarifies antioxidant mechanism of silymarin, brings new information on the antioxidant properties of silymarin. According to the present study, silymarin had effective in vitro antioxidant and radical scavenging activity. It could be used in the pharmacological and food industry because of its antioxidant properties.
Introduction
Oxygen, an element indispensable for life, can under certain circumstances, adversely affect the human body. It is produced by plants during photosynthesis, and is necessary for aerobic respiration in animals. The oxygen consumption inherent in cell growth leads to the generation of a series of reactive oxygen species (ROS). They are continuously produced by the body's normal use of oxygen such as respiration and some cell mediated immune functions. ROS include free radicals such as superoxide anion radicals (O2. − ), hydroxyl radicals (OH·) and non free-radical species such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) Citation1, Citation2, Citation3. ROS are continuously produced during normal physiologic events and can easily initiate the peroxidation of membrane lipids, leading to the accumulation of lipid peroxides. ROS at physiological concentrations may be required for normal cell function. They are also capable of damaging crucial biomolecules such as nucleic acids, lipids, proteins, polyunsaturated fatty acids and carbohydrates and may cause DNA damage that can lead to mutations. If ROS are not effectively scavenged by cellular constituents, they can stimulate free radical chain reactions subsequently damaging the cellular biomolecules such as proteins, lipids and nucleic acids and finally they lead to disease conditions [Citation4,Citation5]. ROS have been implicated in more than 100 diseases [Citation5,Citation6].
All aerobic organisms have antioxidant defences, including antioxidant enzymes and antioxidant food constituents to remove or repair the damaged molecules. Antioxidant compounds can scavenge free radicals and increase shelf life by retarding the process of lipid peroxidation, which is one of the major reasons for deterioration of food and pharmaceutical products during processing and storage [Citation7]. Antioxidants are often added to foods to prevent the radical chain reactions of oxidation, and they act by inhibiting the initiation and propagation step leading to the termination of the reaction and delay the oxidation process Citation10, Citation11, Citation12. At the present time, the most commonly used antioxidants are BHA, BHT, propylgallate and tert-butyl hydroquinone. Besides that BHA and BHT are restricted by legislative rules because of doubts over their toxic and carcinogenic effects [Citation13].Therefore, there is a growing interest on natural and safer antioxidants in food applications, and a growing trend in consumer preferences for natural antioxidants, all of which has given more impetus to explore natural sources of antioxidants Citation14, Citation15, Citation16, Citation17.
Silymarin, a plant derived flavonoid, which is classified as benzopyranone [Citation18], is isolated from the fruits and seeds of the milk thistle (Silymarin marianum) and in reality is a mixture of three structural components: silibinin, silydianine and silychristine () [Citation19]. It has been reported as having multiple pharmacological activities including, hepatoprotectant and anti-inflammatory agent, antibacterial, antiallergic, antimutagenic, antiviral, antineoplastic, antithrombotic agents and vasodilatory actions [Citation20]. One of the important issues regarding silymarin is that it may be accepted as a safe herbal product, since no health hazards or side effects are known in conjunction with the proper administration of designed therapeutic dosages [Citation21].
The aim of this study was to investigate the inhibition of lipid peroxidation in linoleic acid system, ferric ions (Fe3+) reducing antioxidant power assay (FRAP), cupric ions (Cu2+) reducing antioxidant power assay (CUPRAC method), DPPH· scavenging, ABTS√+ scavenging, superoxide anion radical scavenging in the riboflavin/methionine/illuminate system, hydrogen peroxide scavenging and ferrous ions (Fe3+) chelating activities of silymarin. Multiple methods are recommended for measuring antioxidant properties of food or pharmacological materials to better reflect their potential protective effects.
Material and methods
Chemicals
Silymarin, neocuproine (2,9-dimethyl-1,10-phenanthroline), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), nitroblue tetrazolium (NBT), 1,1-diphenyl-2-picryl-hydrazyl (DPPH·), 3-(2-pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine), riboflavin, methionine, linoleic acid, α-tocopherol, polyoxyethylenesorbitan monolaurate (Tween-20) and trichloroacetic acid (TCA) were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). Ammonium thiocyanate was purchased from Merck. All other chemicals used were analytical grade and obtained from either Sigma-Aldrich or Merck.
Total antioxidant activity determination by ferric thiocyanate method
The ferric thiocyanate method was used to evaluate the effect of silymarin and reference antioxidants on preventing peroxidation of linoleic acid described previously [Citation22,Citation23]. A stock solution contained 10 mg of silymarin dissolved in 10 mL distilled water. Silymarin (30 μg/mL) was prepared by diluting the stock solution in 2.5 mL of sodium phosphate buffer (0.04 M, pH 7.0) and these were added to 2.5 mL of linoleic acid emulsion in sodium phosphate buffer (0.04 M, pH 7.0). The linoleic acid emulsion was prepared by homogenising 15.5 μL of linoleic acid, 17.5 mg of Tween-20 as emulsifier, and 5 ml phosphate buffer (pH 7.0). The control was composed of 2.5 mL of linoleic acid emulsion and 2.5 mL, 0.04 M sodium phosphate buffer (pH 7.0). The reaction mixtures (5 mL) were incubated at 37°C in polyethylene flasks. The peroxide levels were determined by reading the absorbance at 500 nm in a spectrophotometer (Shimadzu, UV-1208 UV-VIS Spectrophotometer, Japan) after reaction with FeCl2 and thiocyanate at intervals during incubation. The peroxides formed during linoleic acid peroxidation will oxidize Fe+2 to Fe+3, which forms a complex with thiocyanate that has a maximum absorbance at 500 nm. The assay steps were repeated every 5 h until reaching a maximum. The percent inhibition was calculated at this point (50 h). Solution without silymarin was used as blank samples. Linoleic acid mixture without the addition of sample was used as a control. The percent inhibition of lipid peroxidation in linoleic acid emulsion was calculated by following equation:
in where AC is the absorbance of the control reaction, which contains only linoleic acid emulsion and sodium phosphate buffer. AS is the absorbance of sample in the presence silymarin or others test compounds [Citation24,Citation25].
Ferric cyanide (Fe3+) reducing antioxidant power assay
Reducing power was measured by the direct reduction of Fe3+(CN− )6 to Fe2+(CN− )6 and was determined by absorbance measurement of the formation of the Perl's Prussian Blue complex following the addition of excess Fe3+. For this reason, the ferric reducing antioxidant power (FRAP) method of Oyaizu [Citation26] with slight modification [Citation24] was used to measure the reducing capacity of silymarin. The FRAP method is based on the reduction of (Fe3+) ferricyanide in stoichiometric excess relative to the antioxidants [Citation27]. Different concentrations of silymarin (10–30 μg/mL) in 0.75 mL of distilled water were mixed with 1.25 mL of 0.2 M, pH 6.6 sodium phosphate buffer and 1.25 mL of potassium ferricyanide [K3Fe(CN)6] (1%) the mixture was incubated at 50°C for 20 min. After 20 min incubation, the reaction mixture was acidified with 1.25 mL of trichloroacetic acid (10%). Finally, 0.5 mL of FeCl3 (0.1%) added to this solution and the absorbance was measured at 700 nm in a spectrophotometer. Increased absorbance of the reaction mixture indicates grater reduction capability [Citation28,Citation29].
Cupric ions (Cu2+) reducing power-CUPRAC assay
For reducing ability of silymarin, the cupric ions (Cu2+) reducing power method was also used [Citation30,Citation31] described previously. To a test tube were added 0.25 mL CuCl2 solution (0.01 M), 0.25 mL ethanolic neocuproine solution (7.5 × 10− 3 M) and 0.25 mL NH4Ac buffer solution (1 m), followed by mixing; different concentration of silymarin (10–30 μg/mL). Then total volume, adjusted with distilled water to 2 mL and mixed well. Absorbance against a reagent blank was measured at 450 nm after 30 min. Increased absorbance of the reaction mixture indicates increased reduction capability.
Chelating activity on ferrous ion (Fe2+)s
Ferrous ions (Fe2+) chelating activity was measured by inhibition of the formation of Fe2+-ferrozine complex after treatment of test material with Fe2+, following the method of Dinis and co-workers[Citation32]. Fe2+-chelating ability of silymarin was monitored by the absorbance of the ferrous iron-ferrozine complex at 562 nm. Briefly, different concentrations of silymarin (10–20 μg/mL) in 0.4 mL methanol were added to a solution of 0.6 mM FeCl2 (0.1 mL). The reaction was initiated by the addition of 5 mM ferrozine (0.1 mL) dissolved in methanol. Then, the mixture was shaken vigorously and left at room temperature for ten minutes. Absorbance of the solution was then measured spectrophotometrically at 562 nm. The percentage of inhibition of ferrozine-Fe2+ complex formation was calculated by using the formula given bellow:
where AC is the absorbance of control and AS is the absorbance in the presence of silymarin or standards. The control contains only FeCl2 and ferrozine [Citation24,Citation33].
Hydrogen peroxide scavenging activity
The hydrogen peroxide scavenging assay was carried out following the procedure of Ruch and co-workers [Citation34]. The principle of this method is there a decrease in absorbance of H2O2 upon oxidation of H2O2. A solution of 40 mM H2O2 was prepared in 0.1 M phosphate buffer (pH 7.4). Silymarin at the 30 μg/mL concentration in 3.4 mL phosphate buffer was added to 0.6 mL of H2O2 solution (40 mM) and absorbance of the reaction mixture was recorded at 230 nm. A blank solution contained the sodium phosphate buffer without H2O2. The concentration of hydrogen peroxide (mM) in the assay medium was determined using a standard curve (r2:0.9956):
The percentage of H2O2 scavenging by silymarin and standard compounds was calculated using the following equation:
where AC is the absorbance of the control and AS is the absorbance in the presence of silymarin or other scavengers [Citation25,Citation35].
DPPH√ free radical scavenging activity
Radical scavenging capacity of the silymarin was determined and compared to that of BHA, BHT, α-tocopherol and trolox by using the DPPH√, ABTS√+, DMPD√+ and O2√- radical scavenging methods.
The DPPH radical scavenging assay is commonly employed in evaluating the ability of antioxidants to scavenge free radicals. This spectrophotometric assay uses the stable radical, 1,1-diphenyl-2-picrylhydrazyl (DPPH√), as a reagent [Citation36]. The method of Blois [Citation37] previously described by Gülçin [Citation3] was used with slight modifications in order to assess the DPPH· free radical scavenging capacity of silymarin. The DPPH radical absorbs at 517 nm, but upon reduction by an antioxidant or a radical species its absorption decreases. When a hydrogen atom or electron was transferred to the odd electron in DPPH·, the absorbance at 517 nm decreased proportionally to the increases of non-radical forms of DPPH [Citation38]. Briefly, 0.1 mM solution of DPPH· was prepared in ethanol and 0.5 mL of this solution was added to 1.5 mL of silymarin solution in ethanol at different concentrations (10–30 μg/mL). These solutions were vortexed thoroughly and incubated in dark for 30 min. A half hour later, the absorbance was measured at 517 nm against blank samples lacking scavenger. A standard curve was prepared using different concentrations of DPPH·. The DPPH· scavenging capacity was expressed as mM in the reaction medium and calculated from the calibration curve determined by linear regression (r2:0.9974):
The capability to scavenge the DPPH· radical was calculated using the following equation:
where AC is the absorbance at 517 nm of the control reaction (containing all reagents except the test compound) and AS is the absorbance at 517 nm containing the test compound, which c silymarin. The concentration of silymarin providing 50% scavenging of DPPH· (IC50) was calculated from the graph plotted inhibition percentage against silymarin concentration (μg/mL) [Citation39,Citation40]. DPPH·, decreases significantly upon exposure to radical scavengers [Citation17].
ABTS radical cation decolorization assay
ABTS also forms a relatively stable free radical, which decolorizes in its nonradical form [Citation41]. The spectrophotometric analysis of ABTS√+ scavenging activity was determined according to the method of Re and co-workers [Citation42]. In this method, an antioxidant is added to a pre-formed ABTS radical solution and after a fixed time period the remaining ABTS√+ is quantified spectrophotometrically at 734 nm [Citation25]. The ABTS√+ was produced by reacting 2 mM ABTS in H2O with 2.45 mM potassium persulfate (K2S2O8), and allowing the mixture to stand in the dark at room temperature for 6 h before use. Oxidation of the ABTS commenced immediately, but the absorbance was not maximal and stable until more than 6 h had elapsed. The radical cation was stable in this form for more than 2 days in storage in the dark at room temperature. Prior to assay, the solution was diluted in phosphate buffer (pH 7.4) to give an absorbance at 734 nm of 0.700 ± 0.02 in a 1 cm cuvette and equilibrated to 30°C, the temperature at which all the assays were performed. Then, 1 mL of ABTS√+ solution was added to 3 mL of silymarin solutions in ethanol at different concentrations (10–30 μg/mL). The absorbance was recorded 30 min after mixing and the percentage of radical scavenging was calculated for each concentration relative to a blank containing no scavenger. The extent of decolorization is calculated as percentage reduction of absorbance. For preparation of a standard curve, different concentrations of ABTS√+(0.033–0.33 mM) were used. The ABTS√+ concentration (mM) in the reaction medium was calculated from the following calibration curve, determined by linear regression (r2:0.9899):
The scavenging capability of test compounds was calculated using the following equation:
where AC is absorbance of a control lacking any radical scavenger and AS is absorbance of the remaining ABTS√+ in the presence of scavenger [Citation24].
Superoxide anion radical scavenging activity
Superoxide radical scavenging activity of silymarin was performed according to method of Beauchamp and Fridovich [Citation43] described by Zhishen and co-workers [Citation44] with slight modification. Superoxide radicals are generated in riboflavin, methionine, illuminate and assayed by the reduction of NBT to form blue formazan. All solutions were prepared in 0.05 M phosphate buffer (pH 7.8). The photo-induced reactions were performed using fluorescent lamps (20 W). The concentration of silymarin in the reaction mixture was 30 μg/mL. The total volume of the reaction mixture was 3 mL and the concentrations of the riboflavin, methionine and NBT was 1.33–10− 5, 4.46–10− 5 and 8.15–10− 8 M, respectively. The reaction mixture was illuminated at 25°C for 40 min. The photochemically reduced riboflavin generated O2√ − which reduced NBT to form blue formazan. The unilluminated reaction mixture was used as a blank. The absorbance was measured at 560 nm. Silymarin was added to the reaction mixture, in which O2√ − was scavenged, thereby inhibiting the NBT reduction. Decreased absorbance of the reaction mixture indicates increased superoxide anion scavenging activity. The percentage of superoxide anion scavenged was calculated by using the following formula:
where AC is the absorbance of the control and AS is the absorbance in presence of silymarin or standards [Citation16,Citation45,Citation46].
Statistical analysis
The experimental results were performed in triplicate. The data were recorded as mean ± standard deviation and analysed by SPSS (version 11.5 for Windows 2000, SPSS Inc.). One-way analysis of variance ANOVA was performed by procedures. Significant differences between means were determined by Duncan's Multiple Range tests, and p < 0.05 was regarded as significant and p < 0.01 was very significant.
Results
Peroxidation of fatty acids can cause deleterious effects in foods by forming complex mixture of secondary breakdown products of lipid peroxides. Further intake of these foods can cause a number of adverse effects including toxicity to mammalian cells. Lipid peroxidation is thought to proceed via radical mediated abstraction of hydrogen atoms from methylene carbons in polyunsaturated fatty acids [Citation46,Citation47]. Antioxidant activity is defined as the ability of a compound to inhibit oxidative degradation, such as lipid peroxidation [Citation48,Citation49].
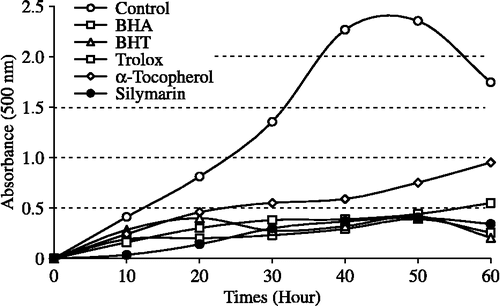
The ferric thiocyanate method measures the amount of peroxide produced during the initial stages of oxidation which are the primary products of oxidation. Silymarin exhibited effective antioxidant activity in the linoleic acid emulsion system. The effect of 30 μg/mL concentration of silymarin on lipid peroxidation of linoleic acid emulsion is shown in and was found to be 82.7%. On the other hand, BHA, BHT, α-tocopherol and trolox exhibited 83.3, 82.1, 68.1 and 81.3% on peroxidation of linoleic acid emulsion at the same concentration, respectively. The autoxidation of linoleic acid emulsion without silymarin or standard compounds was accompanied by a rapid increase of peroxides.
Figure 2. Total antioxidant activities of silymarin and standard antioxidant compounds such as BHA, BHT, α-tocopherol and trolox at the same concentration (30 μg/mL) assayed by ferric thiocyanate method (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).
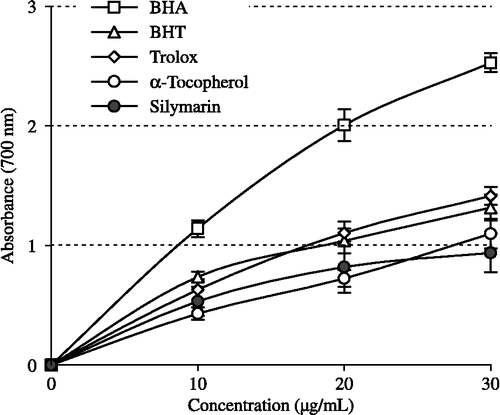
The potassium ferricyanide reduction assay measures the antioxidant effect of any substance in the reaction medium as reducing ability. Silymarin had effective reducing power using the potassium ferricyanide reducing and cupric ions (Cu2+) reducing methods when compared to the standards (BHA, BHT, α-tocopherol and trolox). The Fe3+–Fe2+ transformation ability was investigated in the presence of silymarin using the method of Oyaizu [Citation26]. As can be seen from , silymarin (r2:0.9177) demonstrated powerful Fe3+ reducing ability and these differences were statistically very significant (p < 0.01). The reducing power of silymarin, BHA, BHT, α-tocopherol and trolox increased steadily with increasing concentration of samples. Reducing power of silymarin and standard compounds exhibited the following order: BHA > trolox > BHT > α-tocopherol ≈ silymarin. The results demonstrated that silymarin had marked ferric ions (Fe3+) reducing ability and had electron donor properties for neutralizing free radicals by forming stable products. The outcome of the reducing reaction is to terminate the radical chain reactions that may otherwise be very damaging.
Figure 3. Total Fe3+ → Fe2+ reductive potential of different concentrations (10–30 μg/mL) of silymarin (r2:0.9177) and reference antioxidants; BHA, BHT, α-tocopherol and trolox (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).
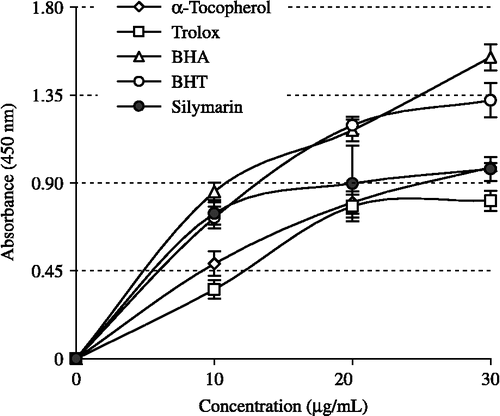
Cupric ions (Cu2+) reducing ability (Cuprac method) of silymarin shown in . It was found a correlation between the cupric ions (Cu2+) reducing ability and silymarin concentrations (r2: 0.9768). Cupric ions (Cu2+) reducing capability of silymarin by Cuprac method was found concentration dependently (10–30 μg/mL). Cupric ions (Cu2+) reducing power of silymarin and standard compounds at the same concentration (30 μg/mL) exhibited the following order: BHA>BHT>α-tocopherol ≈ silymarin>trolox.
Figure 4. Cupric ions (Cu2+) reducing ability of different concentrations (10–30 μg/mL) of silymarin (r2:0.9768), BHA, BHT, α-tocopherol and trolox by Cuprac method (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).
Also, silymarin had also effective chelating effect on ferrous ions (Fe2+). The difference between different concentrations of silymarin (10–20 μg/mL) and the control values was statistically significant (p < 0.01). In addition, silymarin exhibited 85.5% chelation of ferrous ion at 20 μg/mL concentration (r2:0.9068). Ferrous ion chelating effect of the silymarin was compared to that of BHA, BHT, α-tocopherol, trolox and EDTA. On the other hand, the of ferrous ion chelating capacity of the same concentration of EDTA, BHA, BHT, α-tocopherol and trolox were found to be 91.2, 89.3, 84.5, 90.9 and 49.3%, respectively. These results show that the ferrous ion chelating effect of silymarin was statistically similar to EDTA, BHA, BHT, α-tocopherol (p>0.05) but higher than that of trolox (p < 0.01).
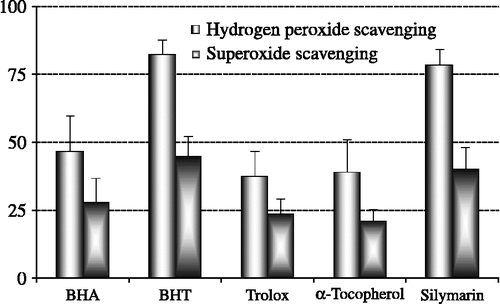
The ability of silymarin to scavenge hydrogen peroxide is shown in and compared with that of BHA, BHT α-tocopherol and trolox as reference compounds. Hydrogen peroxide scavenging activity of silymarin at 30 μg/mL was found to be 78.5%. On the other hand, BHA, BHT, α-tocopherol and trolox exhibited 46.8, 82.5, 39.1 and 37.7% hydrogen peroxide scavenging activity at the same concentration, respectively. These results show that silymarin has an effective hydrogen peroxide scavenging activity. At the above concentration, the hydrogen peroxide scavenging effect of silymarin and four standard compounds decreased in the order of BHT > silymarin > BHA > α-tocopherol ≈ trolox.
Figure 5. Comparison of hydrogen peroxide (H2O2) and superoxide anion radical (O2√ − ) scavenging activities of silymarin and standard antioxidant compounds such as BHA, BHT, (α-tocopherol and trolox at the concentration of 30 μg/mL (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).
DPPH assay has been widely used to evaluate the free radical scavenging effectiveness of various antioxidant substances. In this assay, the antioxidants were able to reduce the stable radical DPPH to the yellow coloured diphenyl-picrylhydrazine. This method is based on the reduction of DPPH in alcoholic solution in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H in the reaction. DPPH is usually used as a reagent to evaluate free radical scavenging activity of antioxidants [Citation26]. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule.
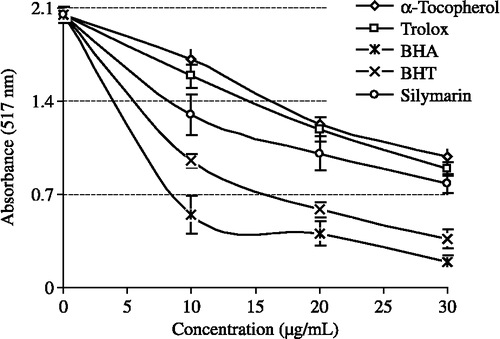
illustrates a significant decrease (p < 0.01) in the concentration of DPPH radical due to the scavenging ability of silymarin (r2:0.9159) and the reference compounds. BHA, BHT, α-tocopherol and trolox were used as references for radical scavenger activity. EC50 values for silymarin, BHA, BHT, α-tocopherol and trolox on the DPPH radical were found as 20.8, 6.1, 10.3, 27.1 and 24.7 μg/mL. A lower EC50 value indicates a higher DPPH free radical scavenging activity.
Figure 6. DPPH free radical scavenging activity of different concentrations (10–30 μg/mL) of silymarin (r2:0.9155) and reference antioxidants; BHA, BHT, α-tocopherol and trolox (BHA: Butylated hydroxyanisole, BHT: Butylated hydroxytoluene; DPPH√: 1,1-diphenyl-2-picryl-hydrazyl free radical).
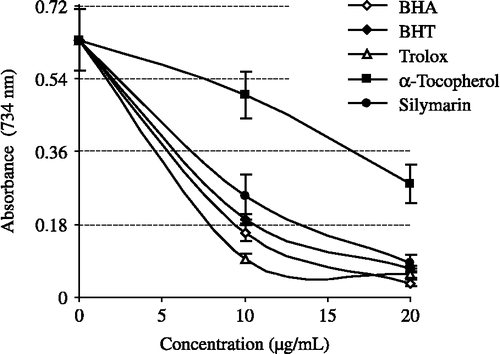
All the tested compounds exhibited effective radical cation scavenging activity. As seen in , silymarin is an effective ABTS√+ radical scavenger in a concentration-dependent manner (10–20 μg/mL, r2:0.9488). EC50 values for silymarin in this assay was 8.62 μg/mL. There is a significant decrease (p < 0.01) in the concentration of ABTS√+ due to the scavenging capacity at all silymarin concentrations. On the other hand, EC50 values for BHA, BHT, α-tocopherol and trolox were found to be 7.50, 8.43, 18.61 and 4.19 μg/mL, respectively.
Figure 7. ABTS radical scavenging activity of different concentrations (10–20 μg/mL) of silymarin (r2:0.9561) and reference antioxidants; BHA, BHT, α-tocopherol and trolox (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene; ABTS√+: 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid).
The inhibition of superoxide radical generation by silymarin is higher than for α-tocopherol and trolox but lower than BHA and BHT. As seen in , the inhibition of superoxide anion radical generation by 30 μg/mL concentration of silymarin was found to be 40.2%. On the other hand, at the same concentration, BHA, BHT, α-tocopherol and trolox exhibited 28.3, 45.2, 21.3 and 23.9% superoxide anion radical scavenging activity, respectively. According to these results, silymarin had higher superoxide anion radical scavenging activity than BHA, α-tocopherol and trolox, but lower than BHT.
Discussion
In present study we demonstrated the antioxidant and radical scavenging mechanism of silymarin by using different in vitro bioanalytic methodologies. It was reported in many studies that the activities of natural antioxidants were closely related to their biofunctionalities. The reduction of chronic diseases, DNA damage, mutagenesis, carcinogenesis and inhibition of pathogenic bacterial growth is often associated with the termination of free radical propagation in biological systems [Citation50]. Antioxidant capacity is widely used as a parameter for medicinal bioactive components. In this study, the antioxidant and radical scavenging activities of silymarin was compared to BHA, BHT, α-tocopherol and its water-soluble analogue trolox. The antioxidant activity of silymarin, BHA, BHT, α-tocopherol and trolox has been evaluated in a series of in vitro tests including DPPH· free radical scavenging, ABTS√+ scavenging, DMPD√+ radical scavenging, total antioxidant activity by the ferric thiocyanate method, reducing power by two methods (Fe3+–Fe2+ transformation and Cuprac assays), scavenging of superoxide anion radical generated in a non-enzymatic system, hydrogen peroxide scavenging and metal chelating on ferrous ions (Fe3+) activities.
Lipid peroxidation consists of a series of free radical-mediated chain reaction processes and is associated with several types of biological damage. The ferric thiocyanate method measures the amount of peroxide produced during the initial stages of oxidation which is the primary product of lipid oxidation. In this assay, hydroperoxides produced from linoleic acid added to the reaction mixture, which has oxidized in air during the experimental period, were indirectly measured. Ferrous chloride and thiocyanate react with each other to produce ferrous thiocyanate (red colour) by means of hydroperoxides [Citation51].
Antioxidants can be reductants, and inactivation of oxidants by reductants can be described as redox reactions in which one reaction species is reduced at the expense of the oxidation of the other. The reducing capacity of a compound can be measured by the direct reduction of Fe[(CN)6]3 to Fe[(CN)6]2. Addition of free Fe3+ to the reduced product leads to the formation of the intense Perl's Prussian blue complex, Fe4[Fe(CN− )6]3, which has a strong absorbance at 700 nm. An increase in absorbance of the reaction mixture would indicate an increase in reducing capacity due to an increase in the formation of the complex. There are a number of assays designed to measure overall antioxidant activity, or reducing potential, as an indication of a host's total capacity to withstand free radical stress [Citation52]. The ferric ion reducing antioxidant power (FRAP) assay takes advantage of an electron transfer reaction in which a ferric salt is used as an oxidant [Citation27]. In this assay, the yellow colour of the test solution changes to various shades of green and blue depending on the reducing power of antioxidant samples. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity.
The Cuprac method was developed for a reducing power assay. This method is simultaneously cost-effective, rapid, stable, selective and suitable for a variety of antioxidants regardless of chemical type or hydrophilicity. Moreover, it was reported that the results obtained from in vitro cupric ion (Cu2+) reducing measurements may be more efficiently extended to the possible in vivo reactions of antioxidants. Cuprac chromogenic redox reaction is carried out at a pH (7.0) close to the physiological pH [Citation53] and the method is capable of measuring thiol-type antioxidants such as glutathione and non-protein thiols unlike the widely applied FRAP test, which is non-responsive to -SH group antioxidants [Citation54].
Among the transition metals, iron is known as the most important lipid oxidation pro-oxidant due to its high reactivity. The effective ferrous ion chelators may also afford protection against oxidative damage by removing iron that may otherwise participate in HO√ generating Fenton type reactions.
Ferric ions also produce radicals from peroxides although the rate is tenfold less than that of ferrous ion. Ferrous ion is the most powerful pro-oxidant among the various species of metal ions. Minimizing ferrous ion may afford protection against oxidative damage by inhibiting production of ROS and lipid peroxidation. Ferrozine can quantitatively form complexes with Fe2+ in this method. In the presence of chelating agents, complex formation is disrupted, resulting in a decrease in the red colour of the complex. Measurement of colour reduction therefore allows estimation of the metal chelating activity of the coexisting chelator. Lower absorbance indicates higher metal chelating activity [Citation55].
One measurement of the metal-chelating activity of an antioxidant is based on absorbance measurement of Fe2+–ferrozine complex after prior treatment of a ferrous ion solution with test material. Ferrozine forms a complex with free Fe2+ but not with Fe2+ bound to other chelators; thus a decrease in the amount of ferrozine–Fe2+ complex formed after treatment indicates the presence of antioxidant chelators. The ferrozine–Fe2+ complex produced a red chromophore with absorbance that can be measured at λ562 nm [Citation55].
EDTA is a strong metal chelator; hence, it is used as standard metal chelator agent in this study. Silymarin demonstrates a marked capacity for iron binding, suggesting that its main action as a peroxidation inhibitor may be related to its iron binding capacity. In this assay, silymarin interfered with the formation of the ferrous-ferrozine complex. It suggests silymarin has chelating activity and is able to capture ferrous ion before ferrozine. Silymarin may chelate the ferrous ion with its hydroxyl group. It was reported that compounds with structures containing C–OH and C = O functional groups can coordinate metal ions. Kazazica and co-workers demonstrated that flavonoids, such as kaempferol, chelated cupric ions (Cu2+) and ferrous ions (Fe2+) through the functional carbonyl groups [Citation56]. The compounds with structures containing two or more of the following functional groups: –OH, –SH, –COOH, –H2PO3, C = O, –NR2, –S– and –O– in a favourable structure-function configuration, can show metal chelation activity. In a previous study, it was shown that L-carnitine chelated ferrous ions (Fe2+) through the carbonyl and hydroxyl functional groups [Citation3]. Recently, Fiorucci and co-workers demonstrated that quercetin chelated metal ions in the same way [Citation57].
Hydrogen peroxide is generated in vivo by several oxidase enzymes such as superoxide dismutase. There is increasing evidence that H2O2, either directly or indirectly via its reduction product OH− , acts as a messenger molecule in the synthesis and activation of inflammatory mediators. It can cross membranes and may slowly oxidize a number of compounds. It is used in the respiratory burst of activated phagocytes. The hydrogen peroxide scavenging capacity of silymarin was shown in . Silymarin had effective hydrogen peroxide scavenging activity. It is known that H2O2 is toxic and induces cell death in vitro. Hydrogen peroxide can attack many cellular energy-producing systems. For instance, it deactivates the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase [Citation58].
The free radical chain reaction is widely accepted as a common mechanism of lipid peroxidation. Radical scavengers may directly react with and quench peroxide radicals to terminate the peroxidation chain reactions and improve the quality and stability of food products. Assays based upon the use of DPPH√, ABTS√+ and DMPD√+ radicals are among the most popular spectrophotometric methods for determination of the antioxidant capacity of foods, beverages and vegetable extracts. Both chromogens and radical compounds can directly react with antioxidants. Additionally, DPPH√, ABTS√+ and DHPD√+ scavenging methods have been used to evaluate the antioxidant activity of compounds due to the simple, rapid, sensitive, and reproducible procedures [Citation59].
Chemical assays are based on the ability to scavenge synthetic free radicals, using a variety of radical-generating systems and methods for detection of the oxidation end-point. DPPH√, ABTS√+, DMPD√+ or radical-scavenging methods are common spectrophotometric procedures for determining the antioxidant capacities of components. When an antioxidant is added to the radicals, there is a degree of decolorization owing to the presence of the antioxidants, which reverses the formation of the DPPH√ radical, ABTS√+ and DMPD√+ cation:
These chromogens such as the violet DPPH and DMPD radical and the blue green ABTS radical cation are easy to use, have a high sensitivity, and allow for rapid analysis of the antioxidant activity of a large number of samples. These assays have been applied to determine the antioxidant activity of pure components [Citation60]. In this study, three radical scavenging methods were used to assess the determination of potential radical scavenging activities of silymarin, namely ABTS√+ radical scavenging, DPPH radical scavenging and·superoxide anion radical scavenging activity.
The DPPH· assay is considered a valid and easy assay to evaluate radical scavenging activity of antioxidants, since the radical compound is stable and does not have to be generated as in other radical scavenging assays. When it reacts with hydrogen donors, the DPPH radical is reduced to the corresponding hydrazine; a decrease in absorbance at 517 nm is produced by the addition of the antioxidant [Citation61]. With this method it was possible to determine the antiradical power of silymarin by measuring a decrease in the absorbance of DPPH· at 517 nm. The structure of the silymarin provide a chromophoric system which leads to interference in the DPPH· method currently using the 517 nm wavelength as described above. The absorbance decreased when the DPPH· was scavenged by an antioxidant through donation of hydrogen to form a stable DPPH radical molecule. In the radical form, this molecule had an absorbance at 517 nm, which disappeared after acceptance of an electron or hydrogen radical from an antioxidant compound to become a stable diamagnetic molecule [Citation62]. However, the best known is that phenolic groups stabilize a radical formed on phenolic carbon with their resonance structure. In silymarin molecule, phenolic group has also three hydroxyl unit. An abstraction of a hydrogen atom from phenolic hydroxyl group may be occurred easily.
The most commonly used radical scavenging methods are ABTS√+ and DPPH√. The DPPH free radical (DPPH√) does not require any special preparation, while the ABTS radical cation (ABTS√+) must be generated by enzymes or chemical reactions.
ABTS√+ radicals are more reactive than DPPH radicals and unlike the reactions with DPPH radical, which involve H atom transfer, the reactions with ABTS√+ radicals involve an electron transfer process [Citation63].
Generation of the ABTS radical cation forms the basis of one of the spectrophotometric methods for measurement of the radical scavenging activity of pure substances, aqueous mixtures and beverages. A more appropriate format for the assay is a decolorization technique, in that the radical is generated directly in a stable form prior to reaction with putative antioxidants. The improved technique for the generation of ABTS√+ described here involves the direct production of the blue/green ABTS√+ chromophore through the reaction between ABTS and potassium persulfate. The ABTS radical cation can be prepared employing different oxidants. Results obtained using K2S2O8 as oxidant show that the presence of peroxodisulfate increases the rate of ABTS√+ auto-bleaching in a concentration-dependent manner. ABTS√+ radicals were generated in the ABTS/K2S2O8 system:
where the scission of the peroxodisulphate could take place after the electron transfer. In the presence of excess ABTS, the sulphate radical will react according to
leading to the overall reaction represented by
ABTS√+ radicals are more reactive than DPPH radicals and, unlike the reactions with DPPH radical, which involve H atom transfer, the reactions with ABTS√+ radicals involve electron transfer.
The principle of the DMPD√+ assay is that at acidic pH and in the presence of a suitable oxidant solution, DMPD can form a stable and coloured radical cation (DMPD√+). The UV-visible spectrum of DMPD√+ shows a maximum absorbance at 505 nm. Antioxidant compounds which are able to transfer a hydrogen atom to DMPD√+ quench the colour and produce a decolouration of the solution. This reaction is rapid (less than 10 min) and the end point, which is stable, is taken as a measure of the antioxidative efficiency. Therefore, this assay reflects the ability of radical hydrogen-donors to scavenge the single electron from DMPD√+.
Preliminary experiments show that the choice of oxidant solution and the ratio between the concentration of DMPD√+ and the concentration of the oxidative compound are crucial for the effectiveness of the method [Citation53].
In contrast to the ABTS procedure the DMPD√+ method guarantees a very stable end point. This is particularly important when a large-scale screening is required. It was reported the main drawback of the DMPD√+ method is that its sensitivity and reproducibility dramatically decreased when hydrophobic antioxidants such as α-tocopherol or BHT were used. Hence, these standard antioxidant compounds were not used in this antiradical assay [Citation53].
Superoxide anion plays an important role in the formation of other ROS such as hydrogen peroxide, hydroxyl radical, and singlet oxygen, which induce oxidative damage in lipids, proteins and DNA. Superoxide radicals are normally formed first, and their effects can be magnified because it produces other kinds of free radicals and oxidizing agents. Superoxide anions derived from dissolved oxygen by the riboflavin/methionine/illuminate system will reduce NBT in this system. The photo-induced reactions were performed using 20 W fluorescent lamps. The reactants were illuminated at 25°C for 5 min. The photochemically reduced riboflavin generated O2− √ which reduced NBT to form blue formazan. In this method, superoxide anion reduces the yellow dye (NBT2+) to produce the blue formazan, which is measured spectrophotometrically at 560 nm. Antioxidants are able to inhibit the blue NBT formation [Citation64]. The decrease of absorbance at 560 nm with antioxidants indicates the consumption of superoxide anion in the reaction mixture. The unilluminated reaction mixture was used as a blank. shows the inhibition of superoxide radical generation by 30 μg/mL concentration of silymarin and standards.
Conclusion
A number of beneficial health effects of silymarin have been reported. In our study, silymarin was found to be an effective antioxidant in different in vitro assays including: total antioxidant activity, reducing power, DPPH√, ABTS√+ and O2√ − radical scavenging, hydrogen peroxide scavenging and metal chelating activities when it is compared to standard antioxidant compounds such as BHA, BHT, α-tocopherol and trolox which is a water-soluble analogue of α-tocopherol. Based on the discussion above, silymarin can be used for minimizing or preventing lipid oxidation in pharmaceutical products, retarding the formation of toxic oxidation products, maintaining nutritional quality and prolonging the shelf life of pharmaceuticals.
Acknowledgements
This study was partially supported by Research Fund of Atatürk University. The author is grateful to the Research Fund of Atatürk University for financial support (Project no: 2001/35).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- EO Farombi, and A Fakoya. (2005). Free radical scavenging and antigenotoxic activities of natural phenolic compounds in dried flowers of Hibiscus sabdariffa. L. Mol Nutr Food Res 49:1120–1128.
- İ Gülçin, M Oktay, Öİ Küfrevioğlu, and A Aslan. (2002). Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol 79:325–329.
- İ Gülçin. (2006). Antioxidant and antiradical activities of L-carnitine. Life Sci 78:803–811.
- B Halliwell, and JMC Gutteridge. (1990). Role of free radicals and catalytic metal ions in human disease: An overview. Method Enzymol 186:1–85.
- İ Gülçin, M Oktay, E Kireçci, and Öİ Küfrevioğlu. (2003). Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem 83:371–382.
- H Tanizawa, Y Ohkawa, Y Takino, A Ueno, T Kageyama, and S Hara. (1992). Studies on natural antioxidants in citrus species. I. Determination of antioxidant activities of citrus fruits. Chem Pharm Bull 40:1940–1942.
- B Halliwell. (1997). Antioxidants in human health and disease. Annu Rev Nut 16:33–50.
- LS Lai, ST Chou, and WW Chao. (2001). Studies on the antioxidative activities of Hsian-tsao (Mesona procumbens Hemsl) leaf gum. J Agric Food Chem 49:963–968.
- İ Gülçin, ME Büyükokuroğlu, M Oktay, and Öİ Küfrevioğlu. (2002). On the in vitro antioxidant properties of melatonin. J Pineal Res 33:167–171.
- F Shahidi, PK Janitha, and PD Wanasundara. (1992). Phenolic antioxidants. Crit Rev Food Sci Nut 32:67–103.
- İ Gülçin, İG Şat, Ş Beydemir, M Elmastaş, and Öİ Küfrevioğlu. (2004). Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem 87:393–400.
- İ Gülçin. (2005). The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nut 56:491–499.
- İ Gülçin, M Elmastas, and HY Aboul-Enein. (2007). Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum) assayed by different methodologies. Phytother Res 21:354–361.
- A Moure, JM Cruz, D Franco, JM Dominguez, J Sineiro, H Dominguez, MJ Nunez, and JC Parajo. (2001). Natural antioxidants from residual sources. Food Chem 72:145–171.
- M Oktay, İ Gülçin, and Öİ Küfrevioğlu. (2003). Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm Wiss Technol 36:263–271.
- İ Gülçin, V Mshvildadze, A Gepdiremen, and R Elias. (2006). Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-D-glucopyranosyl)-hederagenin. Phytother Res 20:130–134.
- İ Gülçin. (2007). Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 32:431–438.
- F Fraschini, G Demartini, and D Esposti. (2002). Pharmacology of silymarin. Clin Drug Invest 22:51–65.
- V Koen, and D Walterovab. (2005). Silybin and silymarin-new effects and applications. Biomed Pap 149:29–41.
- K Abascal, and E Yarnell. (2003). The many faces of Silybum marianum (milk thistle). Alt Comp Ther 251–256.
- HZ Toklu, TT Akbay, G Erkanlı, M Yüksel, F Ercan, and G Şener. (2007). Silymarin, the antioxidant component of Silybum marianum, protects against burn-induced oxidative skin injury. Burns 33:908–916.
- İ Gülçin, and A Daştan. (2007). Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities. J Enz Inhib Med Chem 22 (6):685–695.
- İ Gülçin. (2008). In vitro prooxidant effect of caffeine. J Enz Inhib Med Chem 23 (1):149–152.
- İ Gülçin. (2006). Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 217:213–220.
- İ Gülçin, R Elias, A Gepdiremen, and L Boyer. (2006). Antioxidant activity of lignans from fringe tree (Chionanthus virginicus L.). Eur Food Res Technol 223:759–767.
- M Oyaizu. (1986). Studies on product of browning reaction prepared from glucose amine. Jpn J Nut 44:307–315.
- IFF Benzie, and JJ Strain. (1996). The ferric reducing ability of plasma as a measure of ‘antioxidant power’: The FRAP assay. Anal Biochem 239:70–76.
- ME Büyükokuroğlu, İ Gülçin, M Oktay, and Öİ Küfrevioğlu. (2001). In vitro antioxidant properties of dantrolene sodium. Pharmacol Res 44:491–495.
- İ Gülçin, AZ Tel, and E Kirecci. (2007). Antioxidant, antimicrobial, antifungal and antiradical activities of Cyclotrichium niveum (Boiss.) Manden and Scheng. Int J Food Proper in press.
- R Apak, K Güçlü, M Özyürek, SE Karademir, and E Erça. (2006). The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int J Food Sci Nut 57:292–304.
- İ Gülçin. (2008). Measurement of antioxidant ability of melatonin and serotonin by the DMPD and CUPRAC methods as trolox equivalent. J Enz Inhib Med Chem DOI: 10.1080/14756360701626223
- TCP Dinis, VMC Madeira, and LM Almeida. (1994). Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169.
- İ Gülçin, İG Şat, Ş Beydemir, and Öİ Küfrevioğlu. (2004). Evaluation of the in vitro antioxidant properties of extracts of broccoli (Brassica oleracea L.). Ital J Food Sci 16:17–30.
- RJ Ruch, SJ Cheng, and JE Klaunig. (1989). Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10:1003–1008.
- M Elmastas, İ Türkekul, L Öztürk, İ Gülçin, Ö Işıldak, and HY Aboul-Enein. (2006). The antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta). Comb Chem High T Scr 6:443–448.
- M Burits, and F Bucar. (2000). Antioxidant activity of Nigella sativa essential oil. Phytother Res 14:323–328.
- MS Blois. (1958). Antioxidant determinations by the use of a stable free radical. Nature 26:1199–1200.
- J Ancerewicz, E Migliavacca, PA Carrrupt, B Testa, F Bree, R Zini, JP Tillement, S Labidelle, D Guyot, AM Chauvet-Monges, A Crevat, and A Le Ridant. (1998). Structure-property relationships of trimetazidine derivatives and model compounds as potential antioxidants. Free Radical Bio Med 25:113–120.
- İ Gülçin, Ş Beydemir, HA Alici, M Elmastaş, and ME Büyükokuroğlu. (2004). In vitro antioxidant properties of morphine. Pharmacol Res 49:59–66.
- M Elmastaş, İ Gülçin, Ş Beydemir, Öİ Küfrevioğlu, and HY Aboul-Enein. (2006). A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) seeds extracts. Anal Lett 39:47–65.
- A Shirwaikar, A Shirwaikar, K Rajendran, and ISJ Punitha. (2006). In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull 29:1906–1910.
- R Re, N Pellegrini, A Proteggente, A Pannala, M Yang, and C Rice-Evans. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26:1231–1237.
- C Beauchamp, and I Fridovich. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287.
- J Zhishen, T Mengcheng, and W Jianming. (1999). The determination of flavonoid contents on mulberry and their scavenging effects on superoxide radical. Food Chem 64:555–559.
- İ Gülçin, Ş Beydemir, İG Şat, and Öİ Küfrevioğlu. (2005). Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment 34:193–202.
- İ Gülçin, Öİ Küfrevioğlu, M Oktay, and ME Büyükokuroğlu. (2004). Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 90:205–215.
- N Rajapakse, E Mendis, HG Byun, and SK Kim. (2005). Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J Nut Biochem 16:562–569.
- İ Gülçin, HA Alici, and M Cesur. (2005). Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull 53:281–285.
- V Roginsky, and EA Lissi. (2005). Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 92:235–254.
- QY Zhu, RM Hackman, JL Ensunsa, RR Holt, and CL Keen. (2002). Antioxidative activities of oolong tea. J Agric Food Chem 50:6929–6934.
- R Inatani, N Nakatani, and H Fuwa. (1983). Antioxidative effect of the constituents of rosemary (Rosemarinus officinalis L.) and their derivatives. Agric Biol Chem 47:521–528.
- LG Wood, PG Gibson, and ML Garg. (2006). A review of the methodology for assessing in vivo antioxidant capacity. J Sci Food Agric 86:2057–2066.
- R Apak, K Güçlü, M Özyürek, and SE Karademir. (2004). A novel total antioxidant capacity index for dietary polyphenols, vitamin C and E, using their cupric ion reducing capability in the presence of neocuproine: The CUPRAC method. J Agric Food Chem 52:7970–7981.
- D Huang, B Ou, and RL Prior. (2005). The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856.
- E Köksal, and İ Gülçin. (2008). Antioxidant activity of cauliflower (Brassica oleracea L.). Turk J Agric Forest 32:65–78.
- SP Kazazica, V Butkovica, D Srazica, and D Klasinc. (2006). Gas-phase ligation of Fe+ and Cu+ ions with some flavonoids. J Agric Food Chem 54:8391–8396.
- SB Fiorucci, J Golebıowski, D Cabrol-Bass, and S Antonczak. (2007). DFT study of quercetin activated forms ınvolved inantiradical, antioxidant, and prooxidant biological processes. J Agric Food Chem 55:903–911.
- PA Hyslop, DB Hinshaw, WA Halsey, IU Schraufstatter, RD Sauerheber, RG Spragg, JH Jackson, and CG Cochrane. (1988). Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem 263:1665–1675.
- B Özcelik, JH Lee, and DB Min. (2003). Effects of light, oxygen and pH on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method to evaluate antioxidants. J Food Sci 68:487–490.
- JM Awika, LW Rooney, X Wu, RL Prior, and L Cisneros-Zevallos. (2003). Screening methods to measure antioxidant activity of Sorghum (Sorghum bicolor) and Sorghum product. J Agric Food Chem 51:6657–6662.
- LR Fukumoto, and G Mazza. (2000). Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48:3597–3604.
- B Matthäus. (2002). Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem 50:3444–3452.
- C Sanchez-Moreno. (2002). Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Tech Int 8:121–137.
- I Parejo, F Viladomat, J Bastida, A Rosas-Romero, N Flerlage, J Burillo, and C Codina. (2002). Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants. J Agric Food Chem 50:6882–6890.