Abstract
Inhibitory effects of some analgesic and anaesthetic drugs on human erythrocyte glutathione reductase were investigated. For this purpose, human erythrocyte glutathione reductase was initially purified 2139-fold in a yield of 29% by using 2′, 5′-ADP Sepharose 4B affinity gel and Sephadex G-200 gel filtration chromatography. SDS polyacrylamide gel electrophoresis confirmed the purity of the enzyme by sharing a single band. A constant temperature (+4°C) was maintained during the purification process. Diclofenac sodium, ketoprofen, lornoxicam, tenoxicam, etomidate, morphine and propofol exhibited inhibitory effects on the enzyme in vitro using the Beutler assay method.
Ki constants and IC50 values for drugs were determined from Lineweaver-Burk graphs and plotting activity % versus [I] graphs, respectively. The IC50 values of diclofenac sodium, ketoprofen, lornoxicam, propofol, tenoxicam, etomidate and morphine were 7.265, 6.278, 0.3, 0.242, 0.082, 0.0523 and 0.0128 mM and the Ki constants were 23.97 ± 2.1, 22.14 ± 7.6, 0.42 ± 0.18, 0.418 ± 0.056, 0.13 ± 0.025, 0.0725 ± 0.0029 and 0.0165 ± 0.0013 mM, respectively. While diclofenac sodium, ketoprofen, lornoxicam, tenoxicam etomidate and morphine showed competitive inhibition, propofol displayed noncompetitive inhibition.
Introduction
Glutathione (γ-L-glutamyl-L-cysteinylglycine; GSH) has many important functions: It is an antioxidant, is involved in the detoxification of xenobiotics, and serves as a cofactor in isomerization reactions [Citation1]. Glutathion has an important role in the synthesis and degradation of proteins, regulation of enzymes, formation of the deoxyribonucleotid precursors of deoxyribonucleic acid (DNA) and protection of cells against free radicals and reactive oxygen species [Citation2]. Glutathione reductase(GR) catalyzes the reduction of glutathione disulfide (GSSG) at the expense of NADPH:
By maintaining a high ratio of [GSH]/[GSSG], the enzyme enables several vital functions of the cell such as the detoxification of reactive oxygen species as well as protein and DNA biosynthesis. [Citation3]. Decreased glutathione levels have also been reported in several diseases, such as acquired immune deficiency syndrome [Citation4], Parkinson's disease [Citation5] and diabetes [Citation6,Citation7]. A major role of GSH in erythrocytes is prevention of hemoglobin denaturation, preserving the integrity of erythrocyte membrane sulphydryl groups and detoxification of the xenobiotics and reactive oxygen species in red blood cells [Citation8]. GR has been purified from erythrocytes, using different purification procedures. All reported purification procedures involve several chromotographic steps Citation9, Citation10, Citation11, Citation12, Citation13, Citation14. The effects of many drugs on human, sheep and rat erythrocyte GR enzyme activities have been investigated Citation15, Citation16, Citation17, Citation18. However, no reports could be found in the literature on the effects of diclofenac sodium, ketoprofen, lornoxicam, propofol, tenoxicam, etomidate, morphine, metamizol, paracetamole, indomethazin, fentanyl, chirocaine, marcaine, prilocain and articain on human erythrocyte GR.
The aim of this study was purifying human erythrocyte GR and determination of inhibition or activation effects of some analgesic and anesthetic drugs on human erythrocyte GR activities were investigated.
Materials and Methods
Materials
Sephadex G-200, NADPH, GSSG, and protein assay reagents and chemicals for electrophoresis were obtained from Sigma Chem. Co. 2′, 5′-ADP Sepharose-4B was obtained from Pharmacia. All other chemicals used were analytical grade and obtained from either Sigma-Aldrich or Merck.
GR Activity determination
Enzymatic activity was measured by Beutler's method [Citation19] with a Shimadzu Spectrophotometer UV-(1208), at 25°C. The assay system contained 100 mM Tris-HCl buffer pH 8.0, including 0.5 mM EDTA, 3.3 mM GSSG and 0.1 mM NADPH. One enzyme unit is defined as the oxidation of 1 μmol NADPH per min under the assay condition at 25°C.
Preparation of the hemolysate
Fresh human blood samples were collected in tubes containing EDTA, then centrifuged (15 min, 2,500 × g) and plasma and buffy coat (leucocytes) were removed. The packed red cells were washed three times with KCl (0.16 M) and hemolyzed with 5 volume of ice-cold water and then centrifuged (4°C, 10,000 × g, for 30 min) to remove the ghosts and intact cells [Citation16].
Ammonium sulphate precipitation
The hemolysate was subjected to precipitation with ammonium sulphate (between 30% and 70%). Enzyme activity was determined both in the supernatant and in the precipitate for each respective precipitation. The precipitate was dissolved in phosphate buffer (50 mM; pH = 7.0). The resultant solution was clear, and contained partially purified enzyme. Then it dialysed at 4°C in 1 mM EDTA+10 mM K-phosphate buffer (pH 7.5) for 2 h with two changes of buffer [Citation16]. Partially purified enzyme solution was kept at 4°C.
Purification of the Glutathione Reductase
2′, 5′-ADP Sepharose-4B affinity chromatography
Two grams of dried 2′, 5′-ADP Sepharose-4B was used for a column (1 × 10 cm) of 10 mL bed volume. The gel was washed with 300 mL of distilled water to remove foreign bodies and air, suspended in 0.1 M K-acetate+0.1 M K-phosphate buffer (pH 6.0), and packed in the column. After settling of the gel, the column was equilibrated with 50 mM K-phosphate buffer including 1 mM EDTA, pH 6.0, by means of a peristaltic pump. The flow rates for washing and equilibration were adjusted to 20 mL/h. The dialyzed sample obtained previously was loaded onto the 2′, 5′-ADP Sepharose-4B affinity column and the column was washed with 25 ml of 0.1 M K-acetate+0.1 M K-phosphate, pH 6, and 25 mL of 0.1 M K-acetate+0.1 M K-phosphate, pH 7.85. Washing was continued with 50 mM K-phosphate buffer including 1 mM EDTA, pH 7.0, until the final absorbance difference became 0.05 at 280 nm. The enzyme was eluted with a gradient mixture of 0 to 0.5 mM GSH+1 mM NADPH in 50 mM K-phosphate, containing 1 mM EDTA (pH 7.0). Active fractions were collected and dialyzed with equilibration buffer. All of the procedures were performed at 4°C [Citation16].
Sephadex G-200 gel filtration chromatography
Dried Sephadex G-200 (2 g) was used for a 165 mL column (2 × 50 cm) bed volume. The gel was incubated in distilled water at 90°C for 5 h. After removal of the air in the gel, it was loaded onto the column. Flow rate was adjusted to 15 mL/h by means of a peristaltic pump. Then the column was equilibrated with 50 mM Tris-HCl+50 mM KCl buffer, pH 7.0, until the final absorbance difference became zero at 280 nm. The dialyzed sample was mixed with 5% glycerol. The final sample was loaded onto the column and elutions were collected in 2 mL amounts. In each fraction, enzyme activity was determined at 340 nm. Active fractions were collected and stored at − 20°C for testing the enzyme purity by electrophoresis [Citation16].
Protein determination
The protein content in all samples was quantified spectrophotometrically at 595 nm according to Bradford's method [Citation20], using bovine serum albumin as standard.
Sds Polyacrylamide Gel Electrophoresis (Sds-page)
The control of enzyme purity was carried out using Laemmli's procedure [Citation21] with 3% and 8% acrylamide concentrations for running and stacking gel, respectively. Rabbit phosphorylase B (97,400), bovine albumin (66,000), chicken ovalbumin (45,000), and bovine carbonic anhydrase (29,000) were used as standards (Sigma: MW-SDS-200).
In vitro drug effects
In order to determine the effects of some drugs on human GR, following concentrations of corresponding drugs were included in the reaction mediums (final cuvette concentrations). Diclofenac sodium (7.860–15.72 mM), ketoprofen (1.96–11.75 mM), lornoxicam (0.161–0.538 mM), tenoxicam (0.089–0.208 mM), propofol (0.041–0.82 mM), etomidate (0.028–0.123 mM) and morphine(0.00534–0.02136 mM). The enzyme activity was measured and an experiment in the absence of drug was used as control (100% activity). The IC50 values were obtained from activity (%) vs. drug concentration plots.
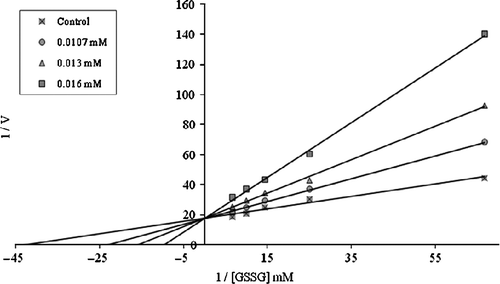
In order to determine Ki constants in the media with inhibitor, the substrate (GSSG) concentrations were 0.015, 0.04, 0.07, 0.10, and 0.15 mM. Inhibitors solutions were added to the reaction medium, resulting in 3 different fixed concentrations of inhibitors in 1 mL of total reaction volume. Lineweaver-Burk graphs [Citation22] were drawn (1/V vs. 1/[S]) and Ki constant were calculated from these graphs. Regression analysis graphs were drawn for IC50 using % inhibition values by a statistical package (SPSS-for windows; version 10.0) on a computer (student t-test; n = 3).
Results
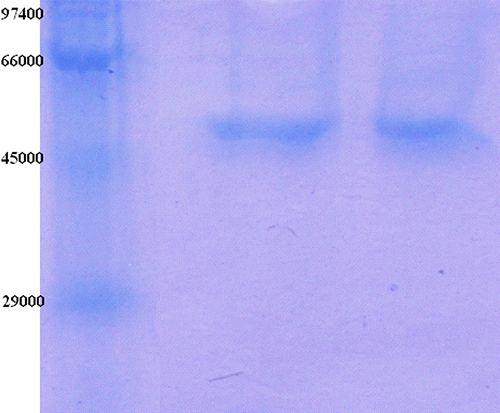
The purification of the enzyme led to a specific activity of 20.75 EU/mg proteins, a yield of 29% and a purification coefficient of 2139 (). SDS polyacrylamide gel electrophoresis was performed after the purification of the enzyme, and the electrophoretic pattern was photographed ().
Table I.. Purification scheme of GR from human erythrocyte.
Figure 1. SDS-PAGE bands of GR (Lane 1: Standards: rabbit phosphorylase B (Mr 97.400), bovine albumin (66.000), chicken ovalbumin (45.000), and bovine carbonic anhydrase (29.000); Lane 2-3: Gel filtration chromatography.
IC50 values of diclofenac sodium, ketoprofen, lornoxicam, propofol, tenoxicam, etomidate and morphine were 7.265, 6.278, 0.3, 0.242, 0.082, 0.0523 and 0.0128 mM and the Ki constants were 23.97 ± 2.1, 22.14 ± 7.6, 0.42 ± 0.18, 0.418 ± 0.056, 0.13 ± 0.025, 0.0725 ± 0.0029 and 0.0165 ± 0.0013 mM, respectively (). (see and for morphine inhibition).
Table II. Ki and IC50 values obtained from regression analysis graphs for GR in the presence of different drugs concentrations.
Discussion
Human GR from erythrocytes was purified in this study by hemolysate preparation, ammonium sulphate precipitation, 2′, 5′-ADP Sepharose 4B affinity chromatography and gel filtration chromatography. The purified preparation was characterized with a specific activity of 20.75 EU/mg proteins, a yield of 29% and a purification coefficient of 2139.These figures tend to validate the procedure used in the study. The SDS-PAGE shows the high purity of the enzyme.
The undesirable biologic effects of oxidative agents, such as free radical and reactive oxygen species (ROS), are eliminated by enzymatic and nonenzymatic antioxidant defense systems. Enzymatic defense is provided by many enzyme systems such as glutathione reductase, glutathione peroxidase, glutathione S-transferase, superoxide dismutase, catalase, aldoketoreductase and DNA repair enzymes [Citation1]. Particularly, GR is essential for the maintenance of cellular glutathione in its reduced form, which is highly nucleophilic for many reactive electrophils [Citation23].
However, to the best of our knowledge, the inhibitory effects of the analgesic and anestethic drugs described on erythrocyte GR have not been studied.
Numerous studies have demonstrated that oxidative stress is a key pathogenic factor in the development of illness complications. In those studies, it was examined effects of ondansetron hydrochloride on antioxidant enzymes. Antioxidant enzymes(glutathione reductase etc.) constitute a supportive team of enzymes which provide defense against the reactive intermediates of dioxygen reduction. These enzymes are cooperative in several aspects [Citation25]. The role of glutathione peroxidase and glutathione reductase in drug resistance must be further unraveled. The inverse relation we showed between the activity of glutathione reductase and the response to cisplatin and cyclophosphamide might indicate a possible role for this enzyme in the detoxification process of the drugs [Citation26].
In order to show inhibitory effects, while the most suitable parameter is the Ki constant, some researchers use the IC50 value. Therefore, in this study, both the Ki and IC50 parameters of these drugs for GR were determined.
It was determined that drugs which have phenolic groups activate glutatione reductase enzyme activity. Similar results were obtained in previous studies Citation15, Citation16, Citation17, Citation18. Similar results were obtained in different study in hepatic rat GR for acetaminophen (paracetamol) [Citation27].
As shown in , the Ki values were 23.97 ± 2.1, 22.14 ± 7.6, 0.42 ± 0.18, 0.418 ± 0.056, 0.13 ± 0.025, 0.0725 ± 0.0029 and 0.0165 ± 0.0013 mM for diclofenac sodium, ketoprofen, lornoxicam, propofol, tenoxicam, etomidate and morphine respectively and the corresponding IC50 values were 7.265, 6.278, 0.3, 0.242, 0.082, 0.0523 and 0.0128 mM, respectively. Ki values and IC50 values show that morphine was the most potent inhibitor followed by etomidate, tenoxicam, propofol, lornoxicam, ketoprofen and diclofenac sodium, respectively.
In this investigation, these analgesic and anaesthetic drugs showed highly inhibitory effects on of human erythrocyte GR activity. Hence, the use of these drugs is undesirable for the enzyme. Besides, fatty acid synthesis may be reduced.
The plasma levels of the drugs used clinically is as follows; ketoprofen ∼0.0783, lornoxicam ∼0.0043, diclofenac sodium ∼0.0471, propofol ∼0.024, tenoxicam ∼0.0119, etomidate ∼0.0175 and morphine ∼0.00762 mM [Citation24]. By taking into account these concentrations, the inhibition data calculated from the plots were found to be ∼0.5%, ∼0.6%, ∼1%, ∼5%, ∼5%, ∼16.73% and ∼29.76%, respectively. According to this data, if it is required to give morphine, etomidate, tenoxicam and propofol to patients, their dosage should be very well controlled to decrease hemolytic and other side effects.
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- A Meisler, and ME Anderson. (1983). Glutathione Ann Rev Biochem 52:711.
- M Gul, FZ Kutay, S Temocin, and O Hanninen. (2000). Cellular and clinical imllications of glutathione. Indian J Exp Biol 38:625.
- RH Schirmer, RL Krauth-Siegel, and GE Schulz. Glutathione reducase New York: John Wiley and Sons Press; (1989). p 553.
- B Akerlund, E Tynell, G Bratt, M Bielentein, and C Lidman. (1997). Nacetylcysteine treatment and the risk of toxic reactions to trimethoprimsulphamethoxazole in primary Pneumocystis carinii prophylaxis in HIVinfected patients. J Infect 35:143.
- P Jenner, and CW Olanow. (1998). Understanding cell death in Parkinson's disease. Ann Neurol 44:72.
- K Yoshida, J Hirokawa, S Tagami, Y Kawakami, Y Urata, and T Kondo. (1995). Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia 38:201.
- S Vijayalingam, A Parthiban, KR Shanmugasundaram, and V Mohan. (1996). Abnormal antioxidant status in impaired glucose tolerance and non-insulindependent diabetes mellitus. Diabet Med 13:715.
- SK Srivastava, and E Beutler. (1970). Glutathione metabolism of the erythrocyte. The enzymic clevage of glutathione-haemoglobin preparations by glutathione reductase. Biochem J 119:353.
- EM Scott, IEW Duncan, and V Ekstrand. (1963). Purification and properties of glutathione reductase of human erythrocytes. J Biol Chem 238:3928.
- GEJ Staal, J Visser, and C Veeger. (1969). Purification and properties of glutathione reductase of human erythrocytes. Biochem Biophys Acta 185:39.
- G Krohne-Ehrich, RH Schirmer, and R Untucht-Grau. (1971). Glutathione reductase of human erythrocytes; Isolation of the enzyme and sequence analaysis of the redox-active peptide. Eur J Biochem 80:65.
- DJ Worthington, and MA Rosemeyer. (1974). Human glutathione reductase: Purification of the crystalline enzyme from erythrocytes. Eur J Biochem 48:167.
- V Boggaram, T Brobjer, K Larson, and B Mannervik. (1979). Purification of glutathione reductase from porcine erythrocytes by the use of affinity chromatography on 2′,5′-ADP-Sepharose 4B and crystallization of the enzyme. Anal Biochem 98:335.
- RR Thıeme, EF Paı, RH Schırmer, and GE Schulz. (1981). 3-dimensional structure of glutathione reductase at 2 a resolution. J Mol Biol 152:763.
- M Erat, and M Ciftci. (2006). Effect of melatonin on enzyme activites of glutathione reductase from human erythrocytes in vitro and from rat erythrocytes in vivo. Eur J Pharmacol 537:59.
- M Senturk, OI Kufrevioglu, and M Ciftci. (2008). Effects of some antibiotics on human erythrocyte glutathione reductase: an in vitro study. J Enz Inhib Med Chem 23:144.
- M Erat, and M Ciftci. (2003). In vitro effects of some antibiotics on glutathione reductase from sheep liver. J Enzm Inhib Med Chem 18:545.
- G Ulusu, M Erat, M Ciftci, H Sakiroglu, and E Bakan. (2005). Purification and characterization of glutathione reductase from sheep liver. Turk J Anim Sci 29:1109.
- E Beutler. Red Cell Metabolism. A Manual of Biochemical Methods. Orlando: Grune and Stratton Inc; (1984). p 134.
- MM Bradford. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248.
- UK Laemmli. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680.
- H Lineweaver, and D Burk. (1934). The determination of enzyme dissocation constants. J Am Chem Soc 56:658–666.
- I Carlberg, and B Mannervik. (1975). Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475.
- SO Kayaalp. Rasyonal tedavi yönünden tıbbi farmakoloji. Ankara: Hacettepe-Tas Yayıncılık (Turkish); (2002).
- N Kaplowitz, TY Aw, and M Ookhtens. (1985). The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol 25:715.
- SM Black, and CR Wolf. (1991). The role of glutathione-dependent enzymes in drug resistance. Pharmacol Ther 51:139.
- PJ O'Brien, MR Slaughter, A Swain, JM Birmingham, RW Greenhill, F Elcock, and PJ Bugelski. (2000). Repeated acetaminophen dosing in rats: adaptation of hepatic antioxidant system. Hum Exper Toxicol 19:277.

![Figure 2. Activity % vs [morphine] regression analysis graphs for human erythrocyte GR in the presence of 5 different morphine concentrations.](/cms/asset/ed19cc48-aa15-45ef-b3d1-c604c5be2d63/ienz_a_318981_f0002_b.gif)