Abstract
The polyphenol oxidase (LsPPO) from a wild edible mushroom Lactarius salmonicolor was purified using a Sepharose 4B-L-tyrosine-p-amino benzoic acid affinity column. At the optimum pH and temperature, the KM and VMax values of LsPPO towards catechol, 4-methylcatechol and pyrogallol were determined as 0.025 M & 0.748 EU/mL, 1.809 × 10− 3 M & 0.723 EU/mL and 9.465 × 10− 3 M & 0.722 EU/mL, respectively.
Optimum pH and temperature values of LsPPO for the three substrates above ranged between the pH 4.5–11.0 and 5–50°C. Enzyme activity decreased due to heat denaturation with increasing temperature. Effects of a variety of classical PPO inhibitors were investigated opon the activity of LsPPO using catechol as the substrate. IC50 values for glutathione, p-aminobenzenesulfonamide, L-cysteine, L-tyrosine, oxalic acid, β-mercaptoethanol and syringic acid were determined as 9.1 × 10− 4, 2.3 × 10− 4 M, 1.5 × 10− 4 M, 3.8 × 10− 7 M, 1.2 × 10− 4 M, 4.9 × 10− 4 M, and 4 × 10− 4 M respectively. Thus L-tyrosine was by far the most effective inhibitor. Interestingly, sulfosalicylic acid behaved as an activator of LsPPO in this study.
Introduction
The collection and consumption of wild edible fungi has traditionally been important to the livelihoods of many people in Northern Eurasia, and is still important, particularly in developing countries. It is a healthy food that can make a useful dietary contribution, being a good source of digestible proteins with low but balanced lipid content, and possessing useful amounts of phosphorus, potassium, selenium, zinc, magnesium, copper and B vitamins [Citation1]. Some dried mushrooms and concentrated extracts are also used for their medicinal properties and as dietary supplements. Thus there is also a strong and increasing commercial interest in fungi, with demand often outstripping local supply [Citation2]. Unfortunately mushrooms are easily prone to browning when they are subjected to forces that can disrupt cellular integrity, such as vibration, rough handling, and ageing [Citation3,Citation4].
Tyrosinases are widely distributed among animals, plants and fungi [Citation5,Citation6]. They are responsible for many biologically essential functions, such as pigmentation, sclerotization, primary immune response and host defense but in mushrooms they are responsible for the browning reactions. Browning reactions are major causes of quality loss during harvesting, post-harvest handling/storage, and processing of fruits, plants and vegetables in food industry [Citation7]. The enzymatic browning causes deterioration of sensory and nutritional quality and affects appearance and organoleptic properties, inactivation of PPO is desirable for preservation of foods [Citation8]. Several methods such as the addition of antioxidants and the exclusion of oxygen as well as thermal processing have been used to inhibit enzymatic browning. For inactivation of PPO, thermal processing has limits like loss of sensory and nutritional quality of food products. Therefore, high-pressure treatment has been considered as an alternative [Citation9,Citation10].
Enzymatic browning via PPO causes deterioration of sensory and nutritional quality and affects appearance and organoleptic properties, thus inactivation of PPO is desirable in food preservation [Citation8]. Several methods such as the addition of antioxidants and the exclusion of oxygen, as well as thermal processing, have been used to inhibit enzymatic browning. However, thermal processing has limits dictated by consequent loss of sensory and nutritional quality of food products. Therefore, high-pressure treatment has been considered as an alternative process of PPO inactivation [Citation9,Citation10].
Here we have purified PPO from the edible wild mushroom, Lactaris salmonicolor, by a single-step affinity procedure, and investigated various enzymic characteristics, including its substrate specificity, kinetics, pH and temperature optima, temperature inactivation, and chemical inhibition.
Materials and methods
Edible mushrooms (Lactarius salmonicolor) used in this study were harvested in the middle of November from a forest near Balikesir in Turkey. The extract of mushroom was prepared as quickly as possible and stored deep-frozen at − 80°C until used. All chemicals used in this study were the best grade available. Enzyme assays were measured with the aid of a Biotek automated recording spectrophotometer. Sepharose 4B,L-tyrosine-p-amino benzoic acid affinity gel which used in this study was synthesized at Balikesir University, Research Center of Applied Sciences (BURCAS/Balikesir, Turkey) in Biology section laboratory [Citation7].
Purification of PPO
All purification steps were carried out at 4°C.The extraction procedure was adopted from Wesche-Ebeling & Montgomery [Citation11]. Firstly, the Lactarius salmonicolor mushrooms were washed with distilled water three times. Secondly, to prepare the crude extract, 50 g of mushrooms were cut quickly into thin slices and homogenized in a Waring blender for 2 min using 100 ml of 0.1 M phosphate buffer, pH:7.3 containing 5% poly(ethylene glycol) and 10 mM ascorbic acid. After filtration of the homogenate through a muslin, the filtrate was centrifuged at 15 000 × g for 30 min, and the supernatant was collected. A crude protein precipitate was made by adding (NH4)2SO4 to 80% saturation. The resulting precipitate was suspended in a minimum volume of 5 mM phosphate bufferand then dialyzed against 5 the same buffer overnight. The enzyme solution was then applied to the Sepharose 4B-tyrosine-p-amino benzoic acid affinity column [Citation7], pre equilibrated with 5 mM phosphate buffer, pH 5.0. The affinity gel was extensively washed with the same buffer before the Lactarius salmonicolor PPO (LsPPO) was eluted with 1 M NaCl, 5 mM phosphate, pH 7,0.
LsPPO activity
Enzyme activity was determined, using different mono- or di-phenolic compounds, by measuring the increase in absorbance at 494 nm for 4-methylcatechol and 500 nm for all other substrates [Citation12] respectively, in a Biotek automated recording spectrophotometer. Enzyme activity was calculated from the linear portion of the curve. One unit of PPO activity was defined as the amount of enzyme that causes an increase in absorbance of 0.001 units min− 1 for 1 ml of enzyme at 25°C [Citation7].
Protein determination
Protein was determined by the method of Bradford [Citation13] using bovine serum albumin as a standard. In chromatography, protein was expressed as absorbance at 280 nm.
Polyacrylamide gel electrophoresis
Polyacrylamide gel slab electrophoresis of purified enzyme was carried out according to the method of Laemmli [Citation14].
Enzyme kinetics and substrate specificity
LsPPO activity was assayed using pyrogallol, catechol and 4-methyl catechol as substrates. The rate of reaction was measured as the increase in absorbance at the absorption maxima of the corresponding quinone product for each substrate. One unit of enzyme activity was defined as the amount of enzyme causing a change of 0.001 in absorbance per minute., LsPPO activities were measured with the substrates at varying concentrations (2.0, 4.0, 6.0, 8.0, 10.0, 12.0, and 15.0 mM) under optimum conditions of pH, ionic strength, and temperature. For each substrate, the Michaelis-Menten constant KM and maximum velocity Vmax values were calculated from a plot of 1/V against 1/[S] by the method of Lineweaver and Burk ().
Effect of pH
LsPPO activity as a function of pH was determined using catechol as substrate (0.1 M stock concentration). The buffers used were 0.1 M acetate (pH 4.5–6.0) and 0.1 M phosphate (pH 6.0–9.5)
Effect of temperature
LsPPO activity, as a function of temperature, was determined under standard assay conditions, using temperatures from 20 to 80°C with pyrogallol and catechol as substrates. The desired temperatures were provided by using an ice bath for temperatures under 20°C and a Tempette Junior TE–85 temperature controller attached to the cell-holder of the spectrophotometer for temperatures above 20°C. The reaction mixtures, containing all the reagents except enzyme, were incubated for 5 min at various temperatures, as indicated above. After the purified enzyme extract was added to the incubated reaction mixture, the activity of LsPPO was determined spectrophotometrically. The final reaction mixture contained 0.6 mL of substrate (0.02 M final concentration), 2.3 mL of 0,1 M buffer solution, and 0.1 mL of enzyme solution. Each assay was repeated twice using the same stock of enzyme extract.
Inhibition of LsPPO activity
An aliquot of each inhibitor at various final concentrations was added to the standard reaction solution immediately before the addition of enzyme extract. The concentration of inhibitor (L-cysteine, p-aminobenzenesulfonamide, glutathion and sulfosalicylic acid) giving 50% inhibition (IC50) was determined from a plot of residual activity against inhibitor concentration, with 10 mM catechol as substrate. The control was activity without inhibitor.
Results and discussion
Extraction and purification of LsPPO
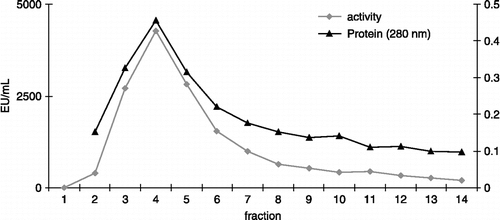
In the past, purification of PPO from different sources has used methods such as Triton X–100 extraction, ammonium sulfate precipitation, dialysis, affinity chromatography, Sephadex G–200 gel filtration chromatography, and Phenyl-Sepharose hydrophobic chromatography [Citation7,Citation15,Citation16]. In the present study LsPPO was purified in one step from a crude 80% (NH4)2SO4 precipitate by affinity chromatography on a Sepharose 4B-L-tyrosine-p-amino benzoic acid affinity column (). This affinity chromatography achieved a 26.6-fold purification, as shown in , This is higher than the 17.2, 10.8, 9.0, 4.9 and 6.5 fold purifications variously described for guava [Citation17], yali pear [Citation18] and yam [Citation19]. Previously, our group had obtained 31.5-fold purification for wild pear (Pyrus elaegrifolia) PPO[Citation20] and a 74-fold purification for mulberry (Morus Alba L.) PPO [Citation7] using the same affinity column.
Figure 2. VMAX and KM values of LsPPO with (a) catechol; (b) 4-methyl catechol; (c) pyrogallol substrates.
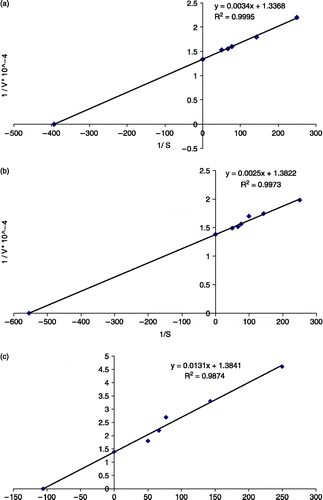
Table I. Purification of polyphenol oxidase from Lactaris salmonicolor.
PPO is widely distributed in all plants. The molecular weight of PPO varies, however, between species [Citation21]. In the present work, the purified LsPPO migrated as a band of approximately 65 kDa (data not shown), upon SDS–polyacrylamide gel electrophoresis. This is the same molecular weight as the PPO isolated from Chinese cabbage [Citation22], mulberry fruit [Citation7] and wild pear [Citation20].
Optimum pH and substrate specificity
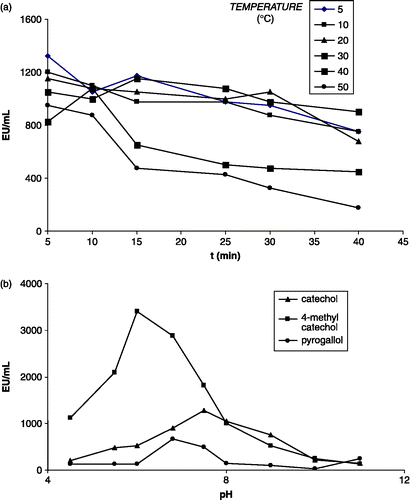
The pH profile of LsPPO activity was determined between pH4.5 and 11.0. As shown in , the optimum pH for maximum LsPPO activity was very dependent on the nature of the substrate used for the assay. Optimum pH values were 6, 7,5 and 7.5 for 4-methyl catechol, catechol, and pyrogallol, respectively. This values were different from those of raspberry pH 8.0 [Citation23], Allium sp. pH 7.5 [Citation24], and mulberry fruit pH 7.0 [Citation7] using catechol as substrate. Different optimum pH values for PPO obtained from different sources are reported in the literature. The optimum pH values are 5.5 for strawberry [Citation11], 6.0 for DeChaunac grape [Citation25], 7.0 for Amasya apple [Citation26], 6.0 for Pyrus elaegrolifia (PePPO) [Citation20]. The optimum pH of mulberry PPO (MPPO) [Citation7] was similar to that of sago log (Metroxylon sagu) pH 7.5 [Citation27] using pyrogallol as substrate.
Optimum temperature & thermal inactivation
To investigate the temperature dependence of LsPPO, catechol was used as a substrate. A temperature range of 5–50°C was investigated and activity monitored after treatment of the enzyme for between 5 and 40 min at each temperature (). It can be seen that at the shortest treatment time (5 minutes) the highest activity was seen at 5°C. Even this short period at higher temperatures led to reduced activity. Whereas activity was reasonably maintained after 40 min of incubation at temperatures of ≤ 30°C, significant inactivation became apparent at 40°C, and more so at 50°C. In the literature, the maximum activity of various PPO species, using catechol as substrate, has been reported as being at 22°C for potato [Citation28], 40°C for Chinese cabbage [Citation22], 12°C for Ferula sp. [Citation29], and 25°C for artichoke [Citation30].
Enzyme inhibition
A considerable number of inhibitors of PPO-induced browning of fruits and vegetables have been identified. Reducing agents have been widely used, but these may have adverse health effects and can also react with other components in the food system [Citation31]. Another important group of browning inhibitors is comprised of compounds that are structurally analogous to the phenolic substrates of PPO, the inhibitory capabilities of which will depend on the enzyme source and the substrate used [Citation32]. Here we investigated the effects of some classical PPO inhibitors, namely glutathione, L-cysteine, p-aminobenzenesulfonamide, sulfosalicylic acid and L-tyrosine, on the activity of LsPPO using catechol as the substrate. IC50 values of 9.1 × 10− 4, 2.3 × 10− 4 M, 1.5 × 10− 4 M, 3.8 × 10− 7 M, 1.2 × 10− 4 M, 4.9 × 10− 4 M, and 4 × 10− 4 M were obtained for glutathione, p-aminobenzenesulfonamide, L-cysteine, L-tyrosine, oxalic acid, β-mercaptoethanol and syringic acid, respectively. Depending on kinetic analysis, mixed-type inhibition (glutathione), competitive inhibition (p-aminobenzenesulfonamide) and uncompetitive inhibition (L-cysteine, L-tyrosine, oxalic acid and syringic acid) were all seen in this study (). Arslan et. al reported glutathione and L-cysteine as displaying competitive inhibition of mulberry PPO [Citation7] but L-cysteine behaved as an uncompetitive inhibitor for LsPPO. Similarly, sulfosaliycylic acid was an uncompetitive inhibitor in that study, but for LsPPO this compound was an activator.
Table II. Effects of inhibitors on LsPPO.
Conclusions
There are so many kinds of harvested edible mushroom species in the world. Several mushrooms are especially tasty and many are rich on nutrients and some of them are also toxic and dangerous for the human health as well. Mushrooms are also easily preserved, and historically have provided additional nutrition over winter. In some parts of Eurasia, especially in Russia and Nordic countries, mushrooms are an important part of the diet. Around six percent of edible species also have medicinal properties. This contribution to human welfare is difficult to assess and has received little attention. The medicinal properties of mycorrhizal fungi have not been well investigated [Citation33]. Edible fungi already play an important role in the lives of many people and more benefits could be achieved for many years. Mushrooms are considered to be a good source of digestible proteins, and while the lipid content is low, the main classes of lipid compounds are represented including phospholipids, sterols, sterol esters, mono-, di- and triglycerides as well as free fatty acids [Citation1].
The principal enzyme responsible for the browning reactions is a binuclear copper containing enzyme, polyphenol oxidase (PPO; E.C 1.14.18.1), which uses molecular oxygen to catalyze the o-hydroxylation of monophenols to o-diphenols and their further oxidation lead to react with endogenous amino acids and proteins to form complex brown pigments and fort this the colour of mushrooms after processing is strongly influenced by the activity of polyphenoloxidase. The browning of mushrooms might also be caused by the action of bacteria and mold on the mushroom. Pseudomonas tolaasii is regarded as a normal constituent of the microflora of the mushroom bed which could produce a metabolite toxic compounds to mushrooms under certain conditions. The infection appears as a brown injury on mushrooms tissues [Citation34,Citation35]. According to this, wild edible fungi provide a source of food and income benefits to people, for this purpose preventing the food quality of this type of mushroom attempts are being made to investigate the purification polyphenol oxidase activity and its some kinetic properties on Lactaris salmonicolor which is commonly used as a food source in Turkey in the middle of November in Balikesir.
LsPPO has the same apparent mass as most other reported PPO species. Its pH optimum is near neutral (pH 6.0–7.5), and like many other PPO enzymes it is, to some extent, substrate-dependent. Comparing catechol, 4-methyl catechol and pyrogallol the KM and VMax values of LsPPO towards these three substrates were determined as 0.025 M & 0.748 EU/mL, 1.809 × 10− 3 M & 0.723 EU/mL, and 9.465 × 10− 3 M & 0.722 EU/mL, respectively. Its pH optimum is unusually low and shows evidence of sensitivity to thermal denaturation with increasing temperature (, ). Inhibition studies indicate a particularly marked inhibitory potential of L-tyrosine (). Further characterisation of purified fungal PPO enzymes, like LsPPO, may lead to the identification of the most suitable strategies for inhibition of PPO-mediated browning reactions and loss of quality in commercial mushroom processing.
Acknowledgements
The authors would like to thank the group researchers Murat Sayın, Aysegul Sahin and Semra Isık for their technical laboratory support, and also Balikesir University, Research Center of Applied Sciences (BURCAS / Balikesir, Turkey) for providing the research facilities. We also thank Dr. Malcolm Lyon (University of Manchester. Paterson Institute for Cancer Research) for his invaluable contribution to this paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- JE Smith, NJ Rowan, and R Sullivan. (2002). Medicinal mushrooms: a rapidly developing area of biotechnology for cancer therapy and other bioactivities (review). Biotechnol Lett 22:1839–1845.
- ER Boa. How do local people make use of wild edible fungi? Personal narratives from Malawi. In: IR Hall, Y Wang, A Zambonelli, and E Danell. editors. Edible ectomycorrhizal mushrooms and their cultivation. Proceedings of the second international conference on edible ectomycorrhizal mushrooms. July 2001, Christchurch. CD-ROM. Christchurch; New Zealand Institute for Crop and Food Research Limited; (2002).
- Guthrie BD. Studies on the control of bacteria deterioration of fresh, Washed mushrooms (Agaricus bisporus/brunescens); Master Thesis, Pennsylvania State University; 1984.
- Pérez-Gilabert A Morte, M Honrubia, and F García-Carmona. (2001). Partial purification, characterization, and histochemical localization of fully latent desert truffle (terfezia claveryi chatin) polyphenol oxidase. J Agric Food Chem 49:1922–1927.
- CWG Van Gelder, WH Flurkey, and HJ Wichers. (1997). Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 45:1309–1323.
- JR. Whitaker. In: DWS Wong, editor. Food enzymes, structure and mechanisms. New York: Chapman and Hall; (1995). p 271–307.
- O Arslan, M Erzengin, S Sinan, and O Ozensoy. (2004). Purification of mulberry (Morus alba L.) polyphenol oxidase by affinity chromatography and investigation of its kinetic and electrophoretic properties. Food Chem 88 (3):479–484.
- M Hendrickx, I Ludikhuyze Van den Broeck, and C Weemaes. (1998). Effects of high pressure on enzymes related to food quality. Trends Food Sci Technol 9:197–203.
- M Asaka, and R Hayashi. (1991). Activation of polyphenol oxidase in pear fruit by high pressure treatment. Agric Biol Chem 5 (9):2439–2440.
- D Knorr. (1993). Effects of high hydrostatic pressure processes on food safety and quality. Food Technol 47:156–161.
- P Wesche-Ebeling, and MW Montgomery. (1990). Strawberry polyphenol oxidase: extraction and partial characterization. J Food Sci 55:1320–1325.
- JC Espin, M Morales, R Varon, J Tudela, and F Garcia-Canovas. (1995). A continuous spectrophotometric method for determining the monophenolase and diphenolase activities of apple polyphenol oxidase. Anal Biochem 43:2807–2812.
- M Bradford. (1976). A rapid and sensitive method for the quantitaion of microgram quantites of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248.
- UK Laemmli. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
- YM Jiang. (1999). Purification and some properties of polyphenol oxidase of longan fruit. Food Chem 66:75–79.
- CA Weemaes, LR Ludikhuyze, IV Broeck, ME Hendrickx, and PP Tobback. (1998). Activity, Electrophoretic Characteristics And Heat Inactivation Of Polyphenoloxidases From Apples, Avocados, Grapes, Pears And Plums. Lebensmittel-Wissenschaft und-Technologie 31:44–49.
- MA Augustin, HM Ghazali, and H Hashim. (1985). Polyphenoloxidase from guava (Psidium guajava L.). J Agric Food Chem 36:1259–1265.
- H Zhou, and X Feng. (1991). Polyphenol oxidase from yali pear (Pyrus. bretschneideri). J Sci Food Agric 57:307–313.
- FW Martin, and RM Ruberte. (1976). The polyphenol of Dioscorea alata (yam) tubers associated with oxidative browning (varieties). J Agric Food Chem 24 (1):67–70.
- O Arslan, S Sinan, FU Yerlitürk, N Gencer, and Ö Özensoy. (2007). Characterization of polyphenol oxidase from wild pear (Pyrus elaegrifolia). J Food Biochem (Article in press vol 33, issue 2 in 2009) reviewed
- T-M Cheng, P-C Huang, J-P Pan, K-Y Lin, and SJT Mao. (2007). Gel electrophoresis of polyphenol oxidase with instant identification by in situ blotting. J Chromatog B 849 (1–2):331–336.
- T Nagai, and N Suzuki. (2001). Partial purification of polyphenol oxidase from Chinese cabbage Brassica rapa L.. J Agric Food Chem 49:3922–3926.
- EM Gonzalez, B deAncos, and MP Cano. (1999). Partial characterization of polyphenoloxidase activity in raspberry fruits. J Agric Food Chem 47:4068–4072.
- O Arslan, A Temur, and I Tozlu. (1997). Polyphenol oxidase from Allium sp. J Agric Food Chem 45:2861.
- CY Lee, NL Smith, and AP Pennesi. (1983). Polyphenol oxidase from DeChaunac grapes. J Sci Food Agric 34:987–991.
- M Oktay, I Kufrevioglu, I Kocacaliskan, and H Sakiroglu. (1995). Polyphenol oxidase from Amasya apple. J Food Sci 60:495–499.
- L Vomas-Vigyazo. (1981). Polyphenoloxidase and peroxidase in fruits and vegeatables. CRC Critical Reviews Food Sci Nutr 14:44.
- G Matheis, and H-D Belitz. (1977). Studies on enzymic browning of potatoes (Solanum tuberosum). III. Kinetics of potato phenoloxidase (EC 1.14.18.1 monophenol, dihydroxyphenylalanine: Oxygenoxidoreductase). Z Lebensm Unters Forsch 163:191.
- M Erat, H Şakiroglu, and OI Kufrevioglu. (2006). Purification and characterization of polyphenol oxidase from Ferula sp. Food Chem 95:503–508.
- T Aydemir. (2004). Partial purification and characterization of polyphenol oxidase from artichoke (Cynara scolymus L.) heads. Food Chem 87:59–67.
- AJ McEvily, R Iyengar, and WS Otwell. (1992). Inhibition of enzymatic browning in foods and beverages. Crit Rev Food Sci Nutr 32:253–273.
- AM Mayer, and E Harel. (1979). Polyphenol oxidase in plants. Phytochemistry 18:193–215.
- SV Reshetnikov, SP Wasser, and KK Tan. (2001). Higher basidiomycota as a source of Antitumour and immunostimulating polysaccharides. A review. Inter J Med Mushrooms 3:361–394.
- SG Paine. (1919). Studies in bacteriosis II. A brown blotch disease of cultivated mushrooms. Ann Appl Biol 5:206–219.
- DJ Royse, and PJ Wuest. (1980). Mushroom brown blotch. Effects of chlorinated water on disease intensity and bacterial populations in casing soil and pilei. Phytopathology 70 (9):902–905.